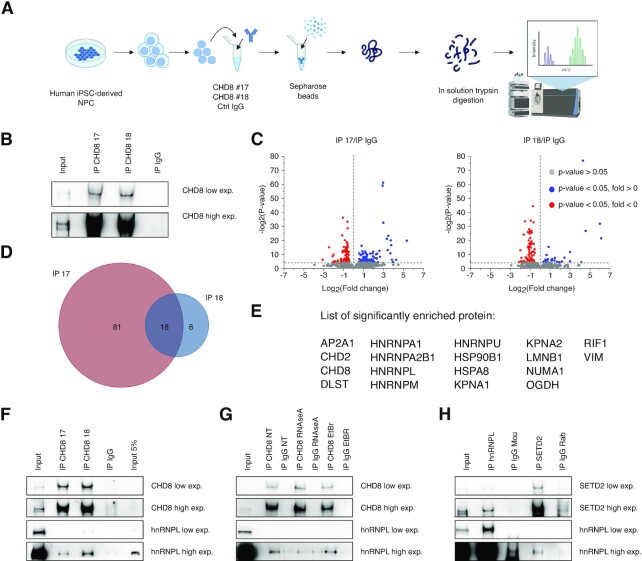
Figure 4.
hnRNPL as a novel CHD8 interactor: bridging altered splicing to H3K36me3 enrichment. (A) Schematic representation of the MS/MS experimental design and approach used in this work (figure created in BioRender.com). Nuclei from hiNPCs (11) were separated from the cytoplasmic fraction. The protein of interest was isolated from the nuclear lysate by specific primary antibodies followed by incubation with Sepharose beads. CHD8 immunoprecipitated proteins were then processed by in solution trypsin digestion prior to MS/MS analysis. (B) Representative western blot images depict immunoprecipitation by endogenous, full-length CHD8 in nuclear extracts by two different antibodies CHD8 NB100-60417 (CHD8 #17) and NB100-60418 (CHD8 #18). A strong, reproducible enrichment compared with Input (Input, 15 μg of nuclear lysate) and rabbit IgG control (IgG) is evident. High exp, 30 s; low exp = 4 s. (C) The volcano plots show CHD8-interacting proteins, significantly differentially enriched compared with IgG controls. Significantly enriched proteins are in blue, significantly depleted proteins in red and non-significant proteins in gray. The threshold for significance is set at a P-value of 0.05. Three independent experiments were averaged and analyzed together for each condition. CHD8, the more represented and enriched peptide with each of the two antibodies, was removed from the plots to optimize visualization of interactors. (D) Venn diagrams represent the overlap between CHD8-interacting proteins identified by CHD8 #17 and CHD8 #18 antibodies. The analysis combines the statistically significant results from three independent biological replicates and two antibodies (Ab #17 A, B, C; Ab #18 A, B, C; see also Supplementary Figure S11). The number of proteins for each condition is indicated. The complete list of proteins is given in Supplementary Table S1. (E) Complete list of the 18 CHD8-interacting proteins identified by CHD8 #17 and CHD8 #18 antibodies. (F) Representative western blot images from co-immunoprecipitation experiments demonstrate interaction between endogenous CHD8 and hnRNPL. Immunoprecipitations were conducted with the two antibodies (IP CHD8 #17 and IP CHD8 #18). A strong, reproducible CHD8 enrichment compared with Input (Input, 15 μg of nuclear lysate, Input 5%, 0.75 μg of nuclear lysate) and rabbit IgG control (IgG) is evident. Co-immunoprecipitation of hnRNPL is clearly visible at high exposure. CHD8 high exp, high exposure = 60 s; CHD8 low exp, low exposure = 20 s. HnRNPL high exp, high exposure = 240 s; CHD8 low exp, low exposure = 75 s. (G) Representative western blot images showing immunoprecipitation of endogenous CHD8 in the nuclear extract with different treatments: RNase A (RNaseA), EtBr or no treatment (NT). CHD8 high exp, high exposure = 30 s; CHD8 low exp, low exposure = 10 s. HnRNPL high exp, high exposure = 60 s; hnRNPL low exp, low exposure = 4 s. (H) Representative western blot images report co-immunoprecipitation between hnRNPL (anti-mouse) and SETD2 (anti-rabbit) antibodies. Endogenous hnRNPL interacts with SETD2, as demonstrated by enrichment over mouse IgG (IP IgG Mou) and INPUT (15 μg of nuclear lysate). Reciprocal co-immunoprecipitation of endogenous SETD2 confirms the interaction, visible at high exposure, compared with IgG controls (IP IgG Rab). SETD2 high exp, high exposure = 20 s; SETD2 low exp, low exposure = 4 s. HNRNPL high exp, high exposure = 40 s; hNRNPL low exp, low exposure = 2 s.