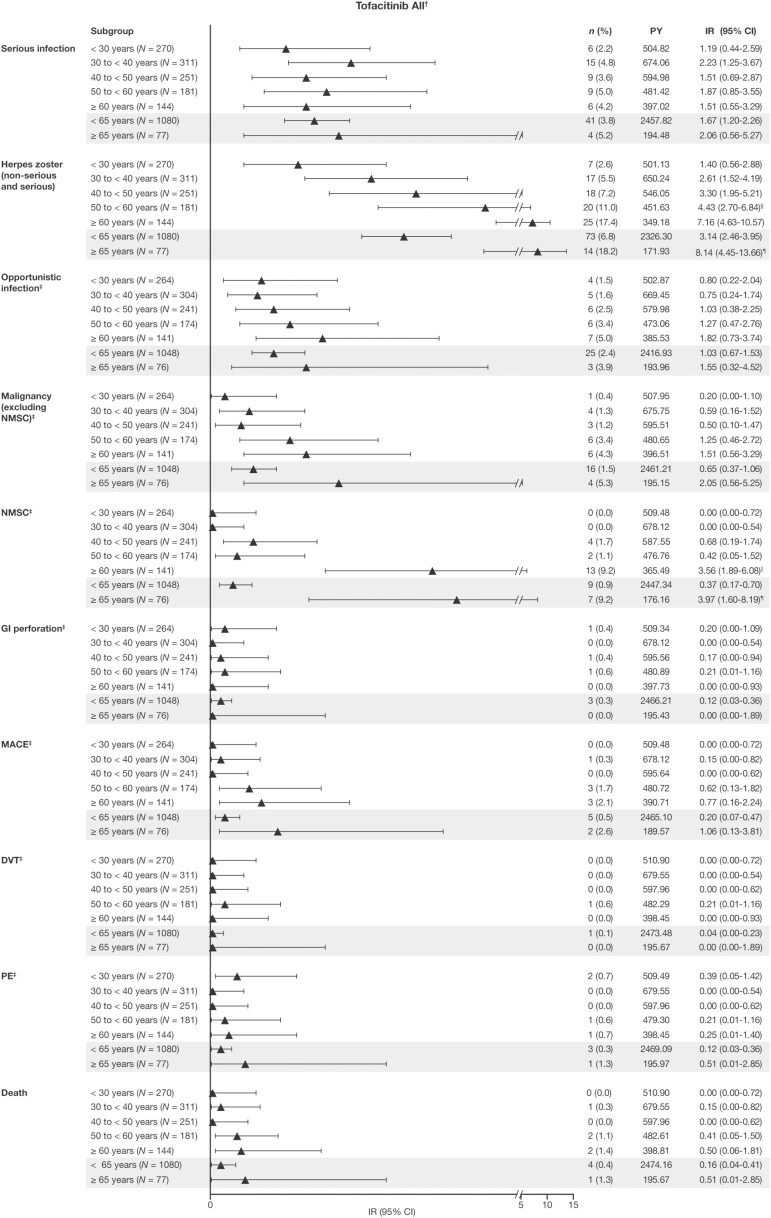
Figure 2.
Safety and AESI reported by age group in the Overall Cohort from the tofacitinib UC clinical program. Data are as of May 27, 2019; database not locked. All malignancy (excluding NMSC), NMSC, MACE, and death events were counted (including those that were outside the 28-day risk period); all other AESI were counted to 28 days beyond the last dose, with the exception of ongoing patients in the OLE study. The IR was defined as the number of unique patients with events per 100 patient-years of exposure. Exact Poisson (adjusted for patient-years) 95% CIs are provided. †Includes patients who received tofacitinib 5 or 10 mg BID in the phase 2 and phase 3 induction and maintenance studies, and the ongoing OLE study, unless stated otherwise. ‡Adjudicated events; the Overall Cohort does not include data from the phase 2 induction study. §The IR was significantly higher than for the group aged < 30 years, based on nonoverlapping 95% CIs. ¶The IR was significantly higher than for the group aged < 65 years, based on nonoverlapping 95% CIs. ††The IR was significantly higher than for the groups ages 18 to < 30, 30 to < 40, 40 to < 50, and 50 to < 60 years, based on nonoverlapping 95% CIs. Abbreviations: AESI, adverse event of special interest; BID, twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate; MACE, major adverse cardiovascular events; N, total number of patients; n, number of patients with the specified event; NMSC, nonmelanoma skin cancer; OLE, open-label, long-term extension; PE, pulmonary embolism; PY, patient-years; UC, ulcerative colitis.