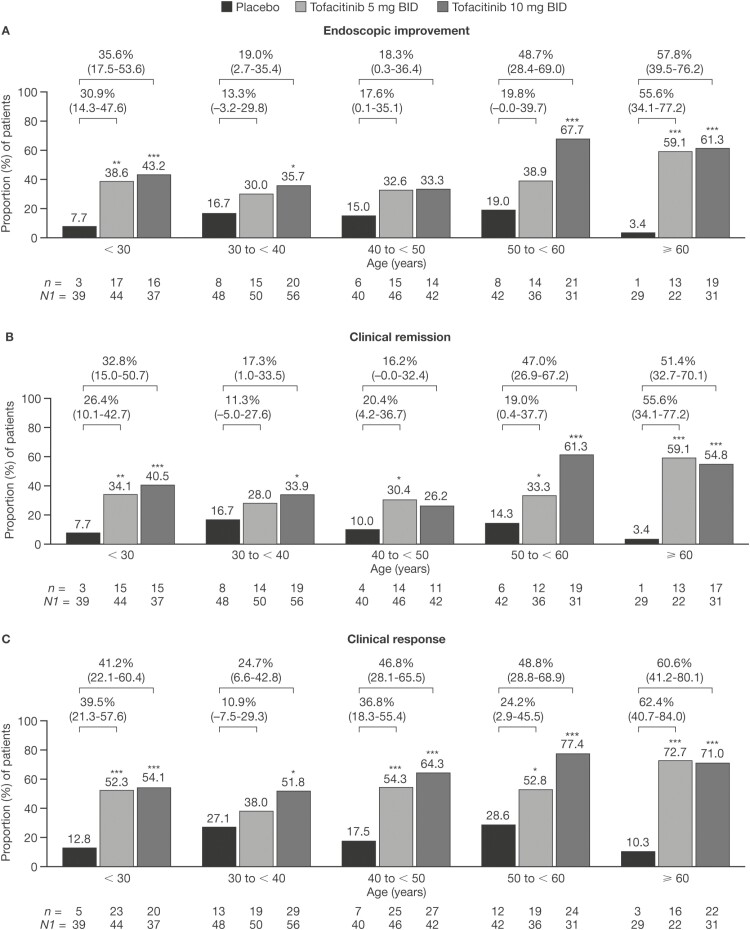
Figure 5.
Proportions of patients receiving placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID who achieved (A) endoscopic improvement, (B) clinical remission, or (C) clinical response at week 52 in the Maintenance Cohort (FAS, NRI). *P < .05; **P < .01; ***P < .001 (P values are from Cochran-Mantel-Haenszel chi-squared test). Values above bars show the difference from placebo (95% CI). Endoscopic improvement was defined as a Mayo endoscopic subscore of 0 or 1. Clinical remission was defined as a total Mayo score of ≤ 2, with no individual subscore exceeding 1 point. Clinical response was defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1. Abbreviations: BID, twice daily; CI, confidence interval; FAS, full analysis set; n, number of patients with the specified response within the given category; N1, number of patients in the specified category with non-missing data; NRI, nonresponder imputation.