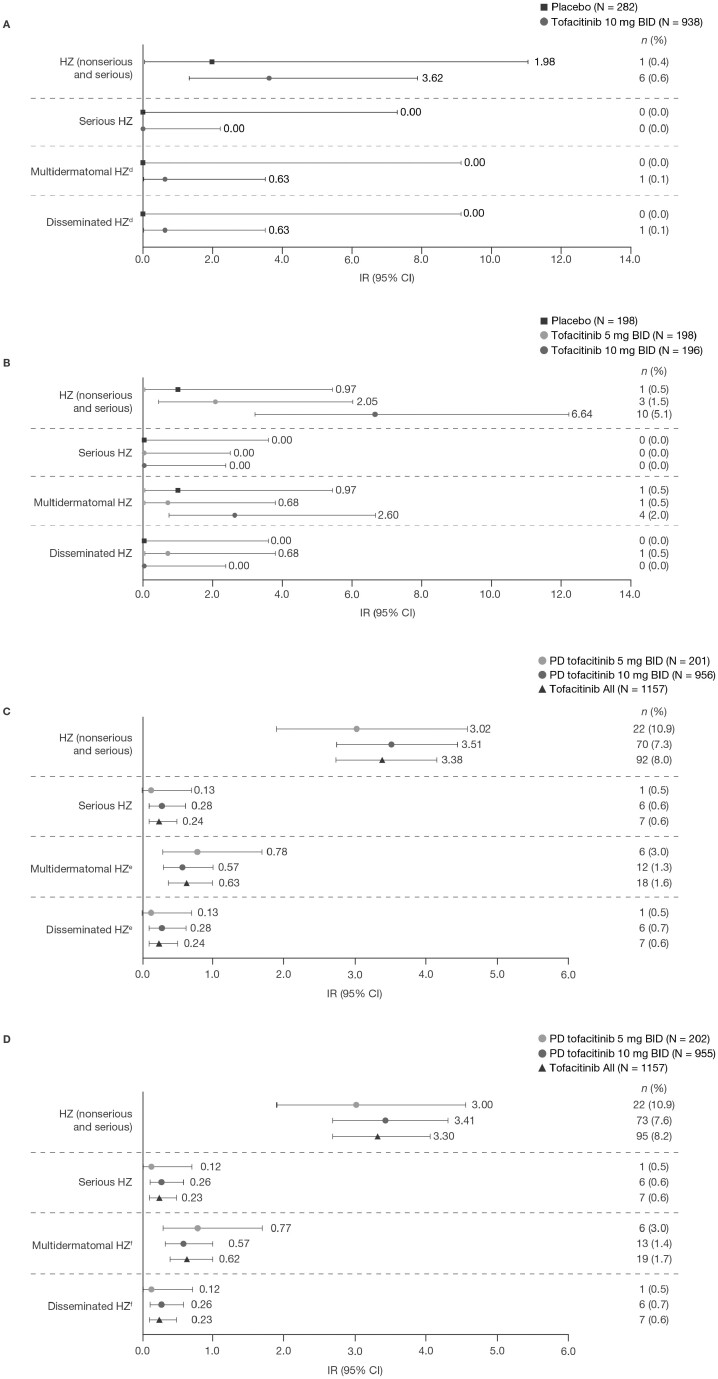
Figure 1.
Proportions and IRs for HZ events in (A) the Induction Cohorta; (B) the Maintenance Cohorta; (C) the Overall Cohort (data as of August 2020)b; and (D) the Overall plus phase 3b/4 Cohort.c
aIncludes data previously reported by Winthrop KL, et al 2018.11bIncludes final data from OCTAVE Open, as of August 24, 2020 (≤7.8 years of exposure). cIncludes final data from OCTAVE Open, as of August 24, 2020, and data from RIVETING, as of February 20, 2020 (≤7.8 years of exposure). dN = 234 and N = 905 for placebo and tofacitinib 10 mg BID, respectively. eN = 923 and N = 1124 for tofacitinib 10 mg BID and Tofacitinib All, respectively. fN = 202, N = 922, and N = 1124 for PD tofacitinib 5 mg BID, PD tofacitinib 10 mg BID, and Tofacitinib All, respectively. Abbreviations: BID, twice daily; CI, confidence interval; HZ, herpes zoster; IR, incidence rate (unique patients with events per 100 PY); N, total number of patients in the analysis; n, number of patients with an event; PD, predominant dose; PY, patient-years.