Abstract
Peripheral neuropathy is a common problem in patients with Parkinson’s disease. Peripheral neuropathy’s prevalence in Parkinson’s disease varies between 4.8–55%, compared with 9% in the general population. It remains unclear whether peripheral neuropathy leads to decreased motor performance in Parkinson’s disease, resulting in impaired mobility and increased balance deficits. We aimed to determine the prevalence and type of peripheral neuropathy in Parkinson’s disease patients and evaluate its functional impact on gait and balance.
A cohort of consecutive Parkinson’s disease patients assessed by movement disorders specialists based on the UK Brain Bank criteria underwent clinical, neurophysiological (nerve conduction studies and quantitative sensory testing) and neuropathological (intraepidermal nerve fibre density in skin biopsy punches) evaluation to characterize the peripheral neuropathy type and aetiology using a cross-sectional design. Gait and balance were characterized using wearable health-technology in OFF and ON medication states, and the main parameters were extracted using validated algorithms.
A total of 99 Parkinson’s disease participants with a mean age of 67.2 (±10) years and mean disease duration of 6.5 (±5) years were assessed. Based on a comprehensive clinical, neurophysiological and neuropathological evaluation, we found that 40.4% of Parkinson’s disease patients presented peripheral neuropathy, with a predominance of small fibre neuropathy (70% of the group). In the OFF state, the presence of peripheral neuropathy was significantly associated with shorter stride length (P = 0.029), slower gait speed (P = 0.005) and smaller toe-off angles (P = 0.002) during straight walking; significantly slower speed (P = 0.019) and smaller toe-off angles (P = 0.007) were also observed during circular walking. In the ON state, the above effects remained, albeit moderately reduced. With regard to balance, significant differences between Parkinson’s disease patients with and without peripheral neuropathy were observed in the OFF medication state during stance with closed eyes on a foam surface. In the ON states, these differences were no longer observable.
We showed that peripheral neuropathy is common in Parkinson’s disease and influences gait and balance parameters, as measured with mobile health-technology. Our study supports that peripheral neuropathy recognition and directed treatment should be pursued in order to improve gait in Parkinson’s disease patients and minimize balance-related disability, targeting individualized medical care.
Keywords: Parkinson’s disease, peripheral neuropathy, wearable health-technology, functional impact
Corrà et al. show that peripheral neuropathy is common in Parkinson’s disease, with a prevalence of approximately 40%, and has a negative impact on gait and balance, as measured with wearable health technology.
Introduction
Parkinson’s disease is a neurodegenerative disorder leading to significant disability and decreased quality of life. With disease progression, motor impairment represents a considerable burden, and gait and balance deficits progressively increase the risk of falls and the management of daily-life activities.1,2 Apart from the hallmark motor symptoms, Parkinson’s disease is considered to be a multi-systemic disorder of the nervous system, and non-motor symptoms have received increasing interest in recent years.3 Among the main features of Parkinson’s disease, a growing number of studies assessing peripheral nerve pathology have recognized the increased prevalence of peripheral neuropathy in the Parkinson’s disease population.4–6
Peripheral neuropathy’s main manifestations are postural instability, loss of peripheral sensation, weakness and pain.7,8 It can be classified into large fibre neuropathy and small fibre neuropathy,9,10 the first diagnosed via the assessment of nerve conduction velocity and amplitude of the electric signal and the latter through a composite evaluation, including the assessment of neurological signs and symptoms, specific neurophysiological tests such as the quantitative sensory testing (QST) and nerve fibre quantitative characterization.11–14 This diagnostic approach must be systematic to increase specificity.11
Peripheral neuropathy was initially considered only in rare genetic forms of Parkinson’s disease,15,16 but a significant number of Parkinson’s disease patients have shown peripheral neuropathy first in case-series and later in multi-centric studies.17–19
The prevalence of peripheral neuropathy in Parkinson’s disease varies depending on the diagnostic methods used and has been shown to be present in up to 55% of Parkinson’s disease patients,6,7,17,18,20 compared with 8–9% in the general population of similar age.21 The association of peripheral neuropathy with Parkinson’s disease has different explanations: (i) it may be linked to Levodopa (L-DOPA) intake,7 proven by a higher prevalence of peripheral neuropathy in patients treated with L-DOPA compared with those not treated with L-DOPA,22 and by a higher prevalence of peripheral neuropathy in patients receiving duodopa or L-DOPA intestinal gel compared with oral L-DOPA22,23; (ii) it may also be an intrinsic feature of Parkinson’s disease, related to loss of small nerve fibres due to, e.g. α-synuclein aggregates (main component of Lewy bodies) not only in the basal ganglia but also in peripheral nerve structures20,24; (iii) concomitant diseases, such as metabolic diseases, autoimmune disorders or infections.11,25
Importantly, peripheral neuropathy in Parkinson’s disease could increase the disability of those affected, leading to additional motor dysfunction,19 higher risk of falls and injuries,26 and worsening of the global functional mobility. Mobility can be evaluated via wearable health-technology, which provides objective and quantitative measures of movements, with a precise estimation of spatio-temporal parameters, allowing high sensitivity, accuracy and reproducibility.27 In fact, the use of wearable health-technology for the assessment of peripheral neuropathy in Parkinson’s disease may provide complementary information to clinical and conventional lab-based assessment tools.28
In order to clarify if peripheral neuropathy has a functional impact on gait and balance in Parkinson’s disease,28 we specifically aimed to: (i) investigate prevalence and types of peripheral neuropathy in Parkinson’s disease with a comprehensive assessment of clinical, neurophysiological and neuropathological evaluation; and (ii) determine whether peripheral neuropathy contributes to impaired mobility in Parkinson’s disease using wearable health-technology.
Materials and methods
Study participants and Parkinson’s disease assessment
We conducted a cross-sectional study with consecutive Parkinson’s disease participants diagnosed by a movement disorders specialist from Centro Hospitalar Universitário do Porto (CHUPorto) based on the UK Brain Bank criteria,29 and attending the CHUPorto movement disorders outpatient clinic from July 2018 to February 2020. The possibility of cohort enrichment for the purpose of groups comparison from movement disorders specialists not involved in this study was also prespecified. We included patients fully able to understand and cooperate with study procedures (maintenance of general cognitive function and daily activities) and without any relevant gait-impairment health issue other than Parkinson’s disease. Demographic and disease specific variables (disease duration, information on daily dopaminergic intake (LEDD)30 and number of falls, the complete Unified Parkinson’s Disease Rating Scale—UPDRS—at both OFF and ON states) were collected. In addition, cognitive tests [Dementia rating scale (DRS)], non-motor symptoms scale (NMSS) and quality of life questionnaire [Parkinson’s disease questionnaire (PDQ-39)] were performed. The study was approved by the institutional review board of CHUPorto (N/REF 2018.087(076-DEFI/076-CES) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects before participation.
Peripheral neuropathy investigation
Clinical assessment
The presence and severity of clinical signs and symptoms characteristics for peripheral neuropathy were evaluated with the use of two scales. The Neuropathy Impairment Score for Lower Limbs (NIS-LL) included the measurement of muscle strength, tendon reflexes and sensation of touch pressure, vibration and joint position at the lower limbs.31 This scale is age-adjusted such that decreased ankle reflexes were considered normal or absent over the age of 70. Participants with a NIS-LL of 3–5 points for the reflexes and sensory parts were considered having mild neuropathy signs, those achieving 6–8 points as having medium neuropathy signs, and those achieving above 9–10 as having severe neuropathy signs.32
The modified Toronto Clinical Neuropathy Score (mTCNS) was used to collect information about participants’ perception of discomfort and neuropathic symptoms (namely foot pain, numbness, tingling and weakness).33 Participants with a total score of ≥6 points were considered to have symptoms of peripheral neuropathy.34
Neurophysiological assessment
Sensory and motor nerve conduction studies were performed using surface recording electrodes with standard placement. The evaluation was performed in the lower limbs (sural sensory, medial plantar, peroneal motor and tibial motor nerve conduction studies, including F-waves). If any of the previous action potentials were below the normative values, the evaluation extended to the upper limbs (ulnar and median sensory and motor nerve conduction studies and radial sensory nerve conduction studies). If a response was absent for any of the above-mentioned nerves (sensory or motor), nerve conduction studies of the contralateral nerve were performed.
In order to assess small nerve fibres, QST examination using the CASE IV system was used to determine the thermal (cold) and heat-pain thresholds through a multimodal approach.35,36 Stimuli were tested on the lower limb (dorsal foot), usually in the same limb as the nerve conduction studies were performed. The testing algorithms were the 4, 2 and 1 stepping method for cold thresholds and the non-repeating ascending with null stimuli for heat-pain thresholds. Normative data from the CASE IV system were used. If any of the tests showed altered results, i.e. above the 97th percentile, the upper limb (dorsal hand) of the same side was also evaluated.37
Neuropathological assessment
Skin specimens were obtained from all participants not taking anticoagulant medication (n = 87) with a disposable 5-mm circular punch under sterile technique after topical anaesthesia. The anatomical sites of skin biopsies were the lateral side of the distal leg (10 cm above the malleolus) and the proximal thigh (20 cm below the greater trochanter). Fixation and incubation of specimens were performed as previously reported.38 Immunohistochemical labeling was performed on 50-μm frozen sections using rabbit polyclonal protein-gene-product (PGP9.5) antibody (Zytomed systems; 1:250), and appropriate Cyanine 3 (Jackson ImmunoResearch Laboratories; 1:50) as the fluorescent secondary antibody. Density was calculated as the number of intraepidermal nerve fibres (IENF) per length of section (IENF/mm). All the tissue sections were analysed using a Nikon Eclipse E400 fluorescence microscope at ×40 magnification. Two criteria were considered to quantify the presence of nerve fibre loss: the normative distal cut-off values reported in literature, stratified by age and sex39 and the gradient between proximal and distal values of IENF (60% or less IENF in the distal probe, compared with the proximal probe, were considered pathologic).40
To investigate the possible link between peripheral nerve fibre loss and Parkinson’s disease pathology, phospho-α;-synuclein detection was performed with the same skin specimens to determine potentially Parkinson’s disease-driven pathology. Serial cryosections (20-μm) were cut and double-immunofluorescence labelling was performed using PGP9.5 and anti-phospho-synuclein (Biolegend; 1:500) and appropriate Cy3 and Alexa Fluor488 (Biolegend; 1:1000)-conjugated secondary antibodies. Biopsies were evaluated at the same fluorescence microscope and classified as positive if at least one dermal nerve fibre phospho-α;-synuclein-immunoreactive in the entire tissue section.24
Blood peripheral neuropathy’s panel
A case-by-case laboratory work-up was performed by a peripheral neuropathy specialist to screen for peripheral neuropathy’s aetiology. Complete blood count, immunoglobulins, T4 lysozyme and thyroid-stimulating hormone, fasting glucose, glucose tolerance and haemoglobin (HbA1C), electrolytes, erythrocyte sedimentation rate, HIV, hepatitis B and C virus serology, antinuclear antibodies, creatinine, blood urea, liver function tests (alanine transaminase, aspartate aminotransferase, alkaline phosphatase, bilirubin, lactate dehydrogenase, gamma-glutamyl transferase), vitamin B6 and B12 levels, methylmalonic acid. homocysteine and folic acid levels were conducted.11 We first checked vitamin B6 and B12 deficiency and vitamin B6 toxicity.41,42 Vitamin B12 deficiency was considered if vitamin B12 levels were below 191 pg/l, or vitamin B12 levels were <500 pg/l, and methylmalonic acid and/or homocysteine were above cut-off.43
Diagnostic criteria for peripheral neuropathy
Participants were diagnosed with large fibre neuropathy via nerve conduction studies.
Participants were diagnosed with small fibre neuropathy if at least two of the following examinations were abnormal11,13:
The sensory part (items related to pinprick, touch pressure, vibration, joint position) of NIS-LL scale ≥ 1 and/or selected items of the mTCNS ≥ 1 (namely foot pain, numbness, tingling, temperature).
Abnormal warm and/or cooling threshold at the foot assessed by QST (≥97th percentile compared to normative data from age and sex-matched healthy controls).
Reduced IENF compared to normative values,39 and 60% and less IENF in the distal probe, compared with the proximal probe.40
Gait and balance assessment
Gait and balance were assessed during ON and OFF medication. Study participants were first evaluated in their OFF medication states in the morning, at least 12 h after the last dose of L-DOPA, and in their ON medication states, after 1–3 h from taking the first dose of L-DOPA during the same day. Participants were equipped with three synchronized RehaGait inertial measurement units (IMUs, Hasomed GmbH), each containing a tri-axial gyroscope and tri-axial accelerometer with sampling frequencies of 100 Hz. The positions of the IMUs were the lower back and lateral parts of both feet.
Gait was assessed with a 20-m straight walking and a 1080° circular walking test. The latter was conducted around a 1.2-m diameter carpet in both left and right directions.44 The Timed Up and Go (TUG) test was also performed. Postural control was assessed with 30-s trials of each, side-by-side stance on the floor and on foam, with eyes opened and eyes closed, and tandem stance on the floor.
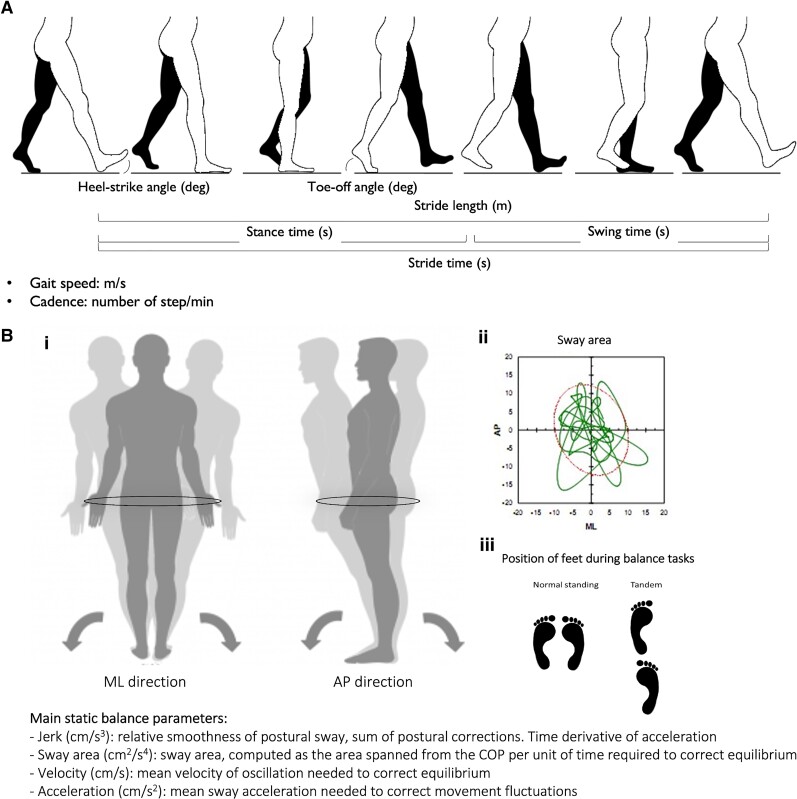
The following gait parameters were extracted using validated algorithms:45–47 from the straight and circular walking data, stride time, cadence, gait variability, gait speed, stride length, heel-strike and toe-off angles; from the TUG data, duration of turns and peak angular velocity during turns; and from the static balance data, jerk in anterior-posterior (AP) and medio-lateral (ML) directions, acceleration in AP and ML directions, velocity in AP and ML directions, as well as sway area.28,48,49 We computed both AP and ML directions, because they were shown to represent different pathologies or compensation strategies of the body.49,50 Explanatory material for the gait and balance parameters used in this study is provided in the Fig. 1.
Figure 1.
Visual representation of gait cycle and postural sway. (A) Gait cycle and main gait parameters extracted from the IMU. (B) Postural sway and main balance parameters extracted from the IMU. (i) Postural sway representation in ML and AP directions and description of main balance parameters. (ii) Representation of sway area on a balance platform. (iii) Position of feet during static balance tasks.
Statistical analysis
Clinical and gait and balance parameters between Parkinson’s disease patients with peripheral neuropathy (PD-PNP) and without peripheral neuropathy (PD-noPNP) were first compared using a t-test or Mann–Whitney U test, as appropriate. Analysis of correlations were used to measure the linear relationship between variables and define the parameters of the final model. Normality of distribution was assessed with Shapiro–Wilk test and variances with Levene’s test. Listwise deletion was applied.
Subsequently, a multivariate analysis of variances was used to evaluate the possible effects of peripheral neuropathy on gait and balance parameters. The analysis was carried out considering PD-PNP and PD-noPNP groups as independent variables and the combined gait and balance features as dependent variables. The analysis was carried out for both OFF and ON medication states separately and controlling for age. For the comparison of different peripheral neuropathy types, a univariate analysis of variance was performed using gait and balance parameters, after controlling for age. The SPSS 25® software package was used. P < 0.05 was considered significant.
Data availability
The data presented in this study are available upon request to the corresponding author.
Results
Parkinson’s disease patients and prevalence of peripheral neuropathy
We assessed 99 consecutive Parkinson’s disease study participants (39.4% women) with a mean age of 67.2 (±10) years and a mean disease duration of 6.5 (±5) years (Table 1). Mean L-DOPA daily dose was 719.1 (±10) mg.
Table 1.
Demographic and clinical characteristics of Parkinson’s disease groups
| PD-PNP (n = 40) | PD-noPNP (n = 59) | P-value | |
|---|---|---|---|
| Sex, n (%) | 18F (45%) | 21F (36%) | 0.350 |
| Age, years | 66.1 (10) | 67.9 (9) | 0.630 |
| Age at disease onset, years | 59.2 (12) | 61.4 (9) | 0.737 |
| Disease duration, years | 7.1 (6) | 6.1 (4) | 0.738 |
| H&Y Stage | 2 | 2 | 0.344 |
| LEDD, mg | 738 (362) | 706.1 (413) | 0.431 |
| UPDRS II ON | 7.3 (4) | 5.2 (4) | 0.004** |
| UPDRS II OFF | 8.8 (4) | 7.5 (5) | 0.095 |
| UPDRS III ON | 15 (9) | 14.9 (8) | 0.849 |
| UPDRS III OFF | 24.1 (11) | 24.7 (10) | 0.754 |
| UPDRS IV | 2.7 (2) | 2.3 (2) | 0.188 |
| NMSS | 29 (21) | 33.2 (27) | 0.638 |
| DRS | 122.4 (15) | 123.2 (16) | 0.603 |
Main demographic and clinical characteristics between PD-PNP and PD-noPNP patients. Values are expressed in mean (standard deviation). Mean comparison (t-test or Mann–Whitney U-test, where appropriate). F = female; H&Y = Hoehn and Yahr stage. **P < 0.01.
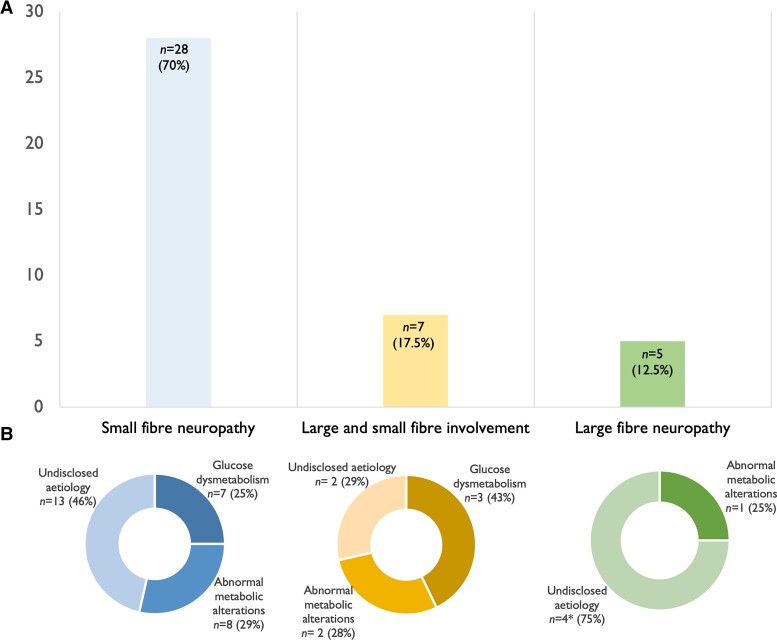
Clinical, neurophysiological and neuropathological assessment showed that 40.4% patients (n = 40) of this Parkinson’s disease cohort presented signs and symptoms allowing peripheral neuropathy diagnosis, with a predominance of small fibre neuropathy (70% of the PD-PNP group) (Fig. 2). The main demographic and clinical characteristics of PD-PNP compared with PD-noPNP participants were not significantly different, except for UPDRS-II in the ON state (P = 0.004) (Table 1).
Figure 2.
Prevalence of peripheral neuropathy in the Parkinson’s disease cohort and related aetiology. (A) Prevalence of peripheral neuropathy types in Parkinson’s disease. Small fibre neuropathy (left column) was observed in 70% (n = 28) of Parkinson’s disease participants; large and small fibre involvement (middle column) represents 17.5% (n = 7) of cases; large fibre neuropathy (right column) was observed in 12.5% (n = 5) of Parkinson’s disease participants. (B) Representation of peripheral neuropathy aetiology for small fibre neuropathy (left), large and fibre neuropathy involvement (middle) and large fibre neuropathy (right) types. Values are expressed as number of subjects (%). *n = 1 patient due to incomplete blood investigation.
Peripheral neuropathy characteristics in Parkinson’s disease patients
Mean NIS-LL and mTCNS scores were 2.3 (±2.7) and 2.2 (±2.3), respectively. A total of 36.3% (n = 36) of the Parkinson’s disease group showed neurological signs of peripheral neuropathy based on the NIS-LL scale cut-off, while 12.1% (n = 12) showed noticeable neuropathic symptoms, according to mTCNS results.
Twelve percent (n = 12) of Parkinson’s disease participants showed axonal large fibre neuropathy, based on nerve conduction studies. No demyelinating features were observed. Sural sensory nerve mean amplitude was 14.5 (±9.3) μV, while superficial peroneal sensory nerve mean amplitude was 13.3 (±7.1) μV. Peroneal motor nerve analysis showed a mean amplitude of 4.9 (±2.1) mV, mean velocity of 47.7 (±6.3) m/s and mean latency of 3.4 (±0.6) ms. QST tests revealed that 21.2% (n = 21) of study participants had impaired sensitivity to cold temperatures and heat-pain, based on temperature thresholds above the 97th percentile.
Mean IENF at the proximal thigh in the entire group was 11.5 (±3.6) and 7.3 (±3.2) at distal leg. A total of 35.3% (n = 35) Parkinson’s disease subjects showed lower IENF at distal leg compared with normative values39 and proximal-distal gradient above 40%. Detailed clinical, neurophysiological and neuropathological results of the peripheral neuropathy investigation are provided in Supplementary Table 1.
Demographic and clinical characteristics of large and small fibre neuropathy participants were not significantly different (Supplementary Table 2). Since large fibre neuropathy often presents with more clinically relevant dysfunction than small fibre neuropathy,20 Parkinson’s disease participants with both small and large fibre neuropathy were included in the large fibre neuropathy group for further analysis.
With regard to aetiology, 25% (n = 10) of the PD-PNP group presented glucose dysmetabolism, while these abnormalities were found in 20.3% (n = 12) of the PD-noPNP group (P = 0.584). Glucose dysmetabolism included patients with diagnosis of Diabetes Mellitus (17.5% of the PD-PNP cohort, n = 7) and patients with HbA1c values ≥6.5% (n = 3).51 Three patients with diabetes showed a multifactorial aetiology of peripheral neuropathy. A total of 27.5% of the group (n = 11) showed alterations in vitamin B6 and B12, methylmalonic acid, homocysteine or folic acid levels. Of this group, 63.3% (n = 7) presented vitamin B12 deficiency, 18.1% (n = 2) showed low B12 values and high B6 values, and 18.1% (n = 2) presented high vitamin B6 levels. Finally, 45% (n = 18) of the PD-PNP group had normal blood results. In these cases with undisclosed aetiology, no significant relation was found with LEDD values. One patient in the PD-PNP group refused to perform the blood test (Fig. 2 and Supplementary Table 3).
Phospho-α;-synuclein deposits were observed in 14.9% of the study cohort (n = 13), mostly in the somatosensory nerve fibres of the subepidermal plexus (n = 11), but also in small nerve fibres around sweat glands (one participant), and in nerve fibres in proximity of the erector pilorum muscle (one participant). Phospho-α;-synuclein deposits location were more frequent at proximal thigh level (61.5% of participants (n = 8) compared to 15.3% (n = 2) with an exclusive distal involvement and 23.2% (n = 3) showing phospho-α;-synuclein deposition at both proximal and distal sites (Supplementary Fig. 1). Phospho-α;-synuclein was present in 30.7% of small fibre neuropathy participants (n = 4). Of this group, two small fibre neuropathy subjects showed diabetes mellitus as main cause of peripheral neuropathy, one participant had abnormal metabolic alterations and one patient presented undisclosed peripheral neuropathy aetiology.
Peripheral neuropathy’s impact on gait and balance
In order to investigate the functional impact of peripheral neuropathy on gait and balance, three additional Parkinson’s disease participants with neuropathic symptoms were subsequently added to the cohort in response to a random selection. A total of 102 (43 PD-PNP and 59 PD-noPNP) participants were therefore included in the following analysis, with a mean age of 67.2 (±10) years and mean disease duration of 6.6 (±5) years. The three additional PD-PNP patients did not relevantly alter the overall cohort characteristics. Gait and balance impairments were firstly analyzed at baseline during the OFF medication state; subsequently, the same functional parameters were investigated in the ON state, to look at the medication effect.
Sensor-based gait parameters
We first performed a preliminary univariate and correlation analysis with all the gait parameters and removed stride time, cadence and gait variability from the final multivariate model, because they did not show a statistical difference between groups and for not satisfying the model’s assumptions.
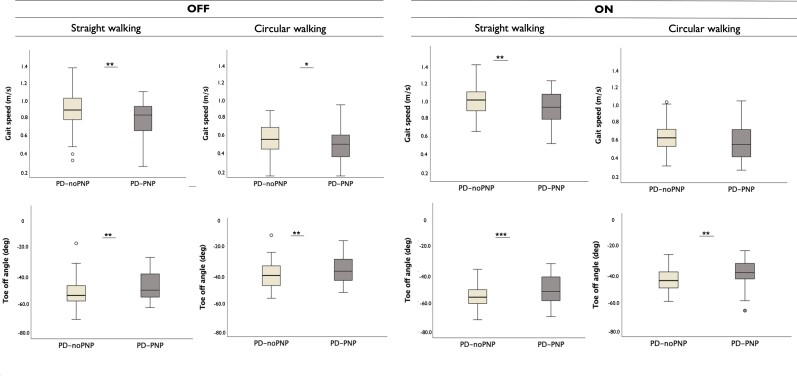
We observed significant differences between the PD-PNP and PD-noPNP groups on the combined dependent parameters of gait across all gait tasks (Table 2). In particular, at baseline, stride length (P = 0.029), gait speed (P = 0.005) and toe-off angles (P = 0.002) were different between groups during straight walking at normal pace. During circular walking, PD-PNP participants showed slower speed (P = 0.019) and smaller toe-off angles (P = 0.007) at OFF state. Peak angular velocity was slower in the PD-PNP group during turns (P = 0.002).
Table 2.
Multivariate analysis of variances of combined gait parameters
| Gait task | Medication state | Value | F-value | df | Error df | P-value |
|---|---|---|---|---|---|---|
| Straight walking | OFF | 0.886 | 2.83 | 4 | 88 | 0.029* |
| ON | 0.84 | 3.904 | 4 | 82 | 0.006** | |
| Circular walking | OFF | 0.888 | 2.861 | 4 | 91 | 0.028* |
| ON | 0.883 | 2.92 | 4 | 88 | 0.026* | |
| Turns | OFF | 0.914 | 4.313 | 2 | 92 | 0.012* |
| ON | 0.944 | 2.765 | 2 | 93 | 0.027* |
Multivariate analysis of variances of combined gait parameters controlled for age during different gait tasks and during OFF and ON medication states. df = degrees of freedom. *P < 0.0; **P < 0.01.
In the ON medication state, the above effects remained, although moderately reduced: during straight walking, all aforementioned parameters remained significantly different between groups; in circular walking and turns, toe-off angles were smaller (P = 0.001) and peak angular velocity lower (P = 0.01) in the PD-PNP group, compared with PD-noPNP (Supplementary Table 4 and Fig. 3).
Figure 3.
Gait parameters distribution in PD-noPNP versus PD-PNP. Distribution of gait speed and toe-off angles parameters during straight and circular walking tasks, in the OFF (left) and ON (right) medication states, between patients with Parkinson’s disease, with (PD-PNP) and without (PD-noPNP) signs of peripheral neuropathy (univariate analysis of variances). *P < 0.05, **P < 0.01, ***P < 0.001.
Sensor-based static balance parameters
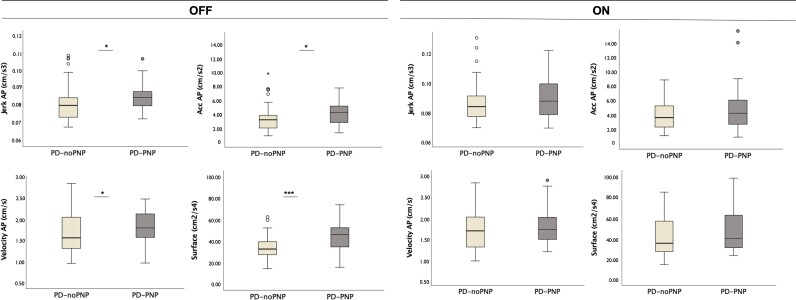
Multivariate analysis of variances on the combined dependent parameters of postural stability was performed for all the balance tasks: side by side stance; tandem stance; and stance with open and closed eyes on a foam surface. No significant differences between groups were observed during static stance on firm surface and stance with open eyes on foam. Notably, only during stance with closed eyes on foam, we found a significant difference between PD-PNP and PD-noPNP on the combined dependent parameters of balance in the OFF medication state, after controlling for age (Table 3). Specifically, jerk in both AP (P = 0.028) and ML (P = 0.001) directions, acceleration AP (P = 0.03), velocity AP (P = 0.034) and sway area (P < 0.001) differed between PD-noPNP and PD-PNP. In the ON medication states, no significant difference was observed between groups (Supplementary Table 5 and Fig. 4).
Table 3.
Multivariate analysis of variances of combined balance parameters
| Balance task | Medication state | Value | F-value | df | Error df | P-value |
|---|---|---|---|---|---|---|
| Side by side stance | OFF | 0.984 | 0.153 | 7 | 64 | 0.993 |
| ON | 0.956 | 0.398 | 7 | 61 | 0.9 | |
| Tandem stance | OFF | 0.595 | 2.436 | 7 | 25 | 0.111 |
| ON | 0.827 | 1.734 | 7 | 58 | 0.119 | |
| Open eyes stance on a foam | OFF | 0.904 | 0.818 | 7 | 54 | 0.577 |
| ON | 0.804 | 1.848 | 7 | 53 | 0.097 | |
| Closed eyes stance on a foam | OFF | 0.633 | 4.216 | 7 | 51 | 0.001** |
| ON | 0.935 | 0.546 | 7 | 55 | 0.796 |
Multivariate analysis of variances of combined balance parameters controlled for age, during different gait tasks and during OFF and ON medication states. df = degrees of freedom. **P < 0.01.
Figure 4.
Balance parameters distribution in PD-noPNP versus PD-PNP. Distribution of the main balance parameters during static balance on a foam surface with closed eyes, in the OFF (left) and ON (right) medication states, between patients with Parkinson’s disease, with (PD-PNP) and without (PD-noPNP) signs of peripheral neuropathy (univariate analysis of variances). *P < 0.05, ***P < 0.001.
Comparison between small and large fibre neuropathy groups
An exploratory analysis was conducted to investigate differences in mobility outcomes between large and small fibre neuropathy types. Both large and small fibre neuropathy types contributed to impaired gait (Supplementary Table 6). In particular, the large fibre neuropathy group showed lower toe-off angles, compared to PD-noPNP during all gait tasks, at both OFF (P = 0.001) and ON (P < 0.001) medication states. In contrast, gait speed was consistently affected by both large and small fibre neuropathy, which was significantly slower compared to PD-noPNP during all gait tasks and all medication states.
With regard to postural stability, the effect of peripheral neuropathy was more pronounced in the large fibre neuropathy group, especially in Jerk ML (P = 0.004), Acceleration ML (P = 0.005) and sway area (P < 0.001) (Supplementary Table 6).
Discussion
In this study, we found that peripheral neuropathy worsens gait and balance in patients with Parkinson’s disease, regardless of its aetiology or the peripheral neuropathy type. Our study shows to the best of our knowledge, for the first time, the impact of peripheral neuropathy on mobility in Parkinson’s disease using wearable health-technology.
Our comprehensive assessment of peripheral neuropathy showed that 40.4% of the Parkinson’s disease population presented peripheral neuropathy, in line with the previously reported prevalence of 4.8–55%.20,52 This variability may be due to differences in population size and use of different methodologies to diagnose peripheral neuropathy.20 For example, previous studies used methodologies directed only to a single type of peripheral neuropathy such as small, autonomic or large fibre neuropathy.5,17,19 Other studies were directed to clinical signs alone versus neurophysiological data.53,54 In our cohort, we comprehensively screened for both large and small fibre neuropathy. Large fibre neuropathy was present in 12.2% of the Parkinson’s disease population. Similar proportions were also observed in previous reports: prevalence of large fibre neuropathy in idiopathic Parkinson’s disease was from 6 to 58%.7 More particularly, a recent systematic review on peripheral neuropathy in Parkinson’s disease addressed an estimated prevalence of large fibre neuropathy of 16.3% from a total of 17 studies and 1376 Parkinson’s disease participants, confirming a higher incidence of peripheral neuropathy in Parkinson’s disease than in the general population.7 With regard to small fibre neuropathy, we found that sensory disturbances were more frequent (35.3% of the Parkinson’s disease cohort) than large fibre neuropathy type.24,55,56 Small fibre neuropathy in Parkinson’s disease was first investigated by different research groups,56,57 who demonstrated reduced small fibre density in Parkinson’s disease subjects. Based on IENF density, the reported prevalence of small fibre neuropathy ranged from 37 to 91%, whereas the pooled estimated prevalence was 56.9% as reported in three studies with a total of 72 participants with Parkinson’s disease.7 A decreased IENF density was also observed in 61% of our cohort. Differently to these previous reports, in our study we used specific and strict criteria for the diagnosis of peripheral neuropathy and its classification, including nerve conduction studies and a comprehensive clinical, QST and neuropathological (IENF density and proximal and distal gradient) criteria to diagnose large and small fibre neuropathy, respectively.11
Our results are also comparable in terms of clinical and nerve conduction studies’ profiles with results from preliminary studies52,53 and studies investigating only one type of peripheral neuropathy.19 We found mostly mild neuropathy signs (83.3%) in the Parkinson’s disease group, confirmed by NIS-LL cut-offs. Strongest neuropathic symptoms confirmed by the mTCNS were observed in only 12.2% of the cohort. These results are in line with previous reports showing higher proportion of altered clinical results in Parkinson’s disease population with peripheral neuropathy compared with Parkinson’s disease participants without peripheral neuropathy and healthy controls.52
We demonstrated that the PD-PNP group did not have a more advanced age and disease duration, compared with the PD-noPNP group. Two reasons may explain the differences between the previous studies and our results: first, peripheral neuropathy and Parkinson’s disease may not be directly related and have independent disease developments; second, it could be that peripheral neuropathy and Parkinson’s disease are related, but evolve distinctively in the central and peripheral nervous systems. Our findings were not in line with previous reports, probably because these studies excluded some peripheral neuropathy aetiologies (such as diabetes mellitus and inflammatory types of peripheral neuropathy), narrowing the scope of the peripheral neuropathy investigation.19,53 The advantage of our study is that we evaluated an unbiased, consecutive series of patients, which may be more representative of the Parkinson’s disease population.
Regarding the aetiology of peripheral neuropathy, 25% of the PD-PNP group was related to glucose dysmetabolism, which is also consistent with published literature.11,58,59 The perecentage of PD-PNP patients with a diagnosis of Diabetes Mellitus was 17.5% (n = 7) compared with 16.9% (n = 10) of the PD-noPNP group (P = 0.943). According to recent surveillance data, the prevalence of Diabetes Mellitus and pre-diabetes forms among adults over 65 years of age varies from 22 to 33%, depending on the diagnostic criteria used.60 Our data showed a total prevalence of 22.2% of patients with glucose dysmetabolism, which is in line with average prevalence of the elderly population. Metabolic alterations are another frequent cause of peripheral neuropathy and were present in less than one-third of the cohort. Low levels of vitamin B12 have already been reported in studies on the aetiology of peripheral neuropathy in the Parkinson’s disease population.20,61 L-DOPA toxicity has been considered a contributing factor to peripheral neuropathy in Parkinson’s disease patients.22 In our cohort we did not find a significant difference in terms of mean LEDDs between Parkinson’s disease patients with and without peripheral neuropathy (P = 0.431). Moreover, methylmalonic acid and homocysteine levels of the PD-PNP group with undisclosed aetiology were within the normal range, and no significant correlation was found with LEDD values. Hence, a causal relationship with L-DOPA was not considered in our group of patients with peripheral neuropathy.22,62 We also found a low prevalence of phospho-α;-synuclein deposits in our cohort, with a higher percentage in the proximal thigh area, compared with the distal leg. Due to the low number of active phospho-α;-synuclein-small fibre neuropathy subjects (n = 4), and in particular of small fibre neuropathy subjects with undisclosed aetiology (n = 1), α;-synuclein deposition was not considered to be directly associated with the pathophysiology of peripheral neuropathy.
Importantly, we found that peripheral neuropathy had a functional impact on gait during all gait tasks. During straight walking, PD-PNP patients presented slower gait speed, shorter stride length and smaller toe-off angles, compared with PD-noPNP patients. This observation is in line with the reduced gait speed and increased risk of falling reported in earlier studies on peripheral neuropathy patients.63–66 Similar results related to gait speed and stride length were also shown by Beaulieu et al.26 in a small cohort of Parkinson’s disease participants with peripheral neuropathy. This promising but still preliminary work had less strict diagnostic criteria, based on signs and symptoms and gait assessment in pressure mapping walkway of only 8 m, which limited the assessment of different gait tasks or specific parameters such as foot angles. We used a more comprehensive peripheral neuropathy assessment protocol and, supported by wearable health technology, we also observed gait deficits during several other gait tasks (such as circular gait and turns), allowing for a more ecological functional assessment.
Smaller toe-off angles during gait were also reported by Hazari et al.67 in a cohort of patients with peripheral neuropathy: the study showed that peripheral neuropathy participants walked with greater knee flexion angles than healthy controls, which may be associated with musculoskeletal changes as a consequence of motor peripheral neuropathy, resulting in weakness and tightness of flexors muscles. Although considering that the patient population studied is different, this study also showed no significant differences in relation to heel strike angles, consistent with our results. These results suggest a more cautious gait in PD-PNP patients (smaller toe-off angle) that, in our case, may be not due to muscular weakness of the extensor muscle of the lower leg (normal heel strike angle) suggesting that, along with motor impairment, sensory and proprioceptive neuropathy may interfere and contribute to such finding.
Overall, our results confirmed that loss of somatosensory function significantly affected gait, both in more ‘automatic’ conditions, such as straight walking at normal pace, as well as during more demanding tasks, such as circular walking and turning. A more impaired gait in the PD-PNP group during straight walking may be related to the inability of the neuromuscular control system to respond to environmental influences when attention is reduced, such as when gait is more automatic.28 Also in the ON medication state, PD-PNP gait remained significantly impaired, compared with PD-noPNP, as also evidenced clinically.
PD-PNP patients also had worse performance during static balance tasks. This was particularly evident in more challenging tasks with closed eyes stance on a foam, where PD-PNP patients presented greater Jerk, acceleration, velocity and sway area values. This observation is best explained by reduced proprioception that cannot be compensated by visual feedback.28 The obvious increased reliance on vision of PD-PNP subjects to have more postural control could also reflect a sensory re-weighting problem. One of the parameters that best discriminated postural control between PD-PNP and PD-noPNP groups was Jerk. Jerk is the sum of active postural corrections to maintain static balance, and represents a measure of smoothness of static balance.47 Studies in peripheral neuropathy patients suggest that increased sway in the AP direction is associated with increased movement in the hip joint (‘hip strategy').50,68–70 We found that most parameters related to static balance, particularly Jerk, acceleration and velocity, were significantly different in the AP direction, confirming the concept that peripheral neuropathy subjects may predominantly show a hip strategy to compensate for the existing balance deficits.71
Significant effects of peripheral neuropathy on static balance were observed only in the OFF medication state, and not in the ON states. This finding suggests that optimizing dopaminergic therapy has a highly relevant effect on static balance in Parkinson’s disease patients suffering from concomitant peripheral neuropathy. The most plausible underlying mechanism may be the dopaminergic system compensation for the lack of information from the somatosensory system, which is caused by peripheral neuropathy. However, a link between the effect of L-DOPA on the basal ganglia system and somatosensory feedback has never been established. We argued that the effect of peripheral neuropathy may be most evident when Parkinson’s disease subjects are not in their best health status and during their best motor performance (in their OFF states), because the lack of dopaminergic compensation typically exacerbates motor impairments in Parkinson’s disease.49 For this reason, it is particularly relevant to ensure an optimal dopaminergic treatment for this specific PD-PNP subgroup.
Regarding the difference between peripheral neuropathy types and their effect on Parkinson’s disease mobility, both peripheral neuropathy types contribute to impaired gait and balance, albeit at different levels. Large fibre neuropathy seemed to affect foot angles and static balance more severely, likely due to more severe reduction of strength and proprioceptive feedback. Small fibre neuropathy may lead to slower speed due to its progressive lack of peripheral sensation.21,72
The results of this study must be viewed in light of some limitations. We evaluated a cohort of Parkinson’s disease participants from a single centre at University hospital. However, our Movement Disorders Outpatient Clinic receives Parkinson’s disease patients at all disease stages, and the consecutive nature of the recruitment, with an extremely high acceptance rate and large sample size, decreasing potential selection biases. Second, the sample size, particularly of large fibre neuropathy patients, was small, not allowing more in-depth peripheral neuropathy types comparison. Further studies with larger cohorts should be performed to understand the effect of peripheral neuropathy on quality of life, including its relation to falls.
Some strengths can also be highlighted: first, the accurate and well-defined diagnosis and clinical evaluation of Parkinson’s disease patients by Movement Disorders’ specialists, even though we acknowledge the possibility of misdiagnosis in the initial phases of the disease (e.g. atypical parkinsonian syndromes); second, we evaluated peripheral neuropathy using a comprehensive assessment with no restriction on peripheral neuropathy types and based on well-defined and complete diagnostic criteria. This enabled our understanding of the overall effect of peripheral neuropathy in Parkinson’s disease and the establishment of some initial steps for its evaluation in clinical routine. Finally, the use of wearable health-technology may be an important new tool for assessing peripheral neuropathy in Parkinson’s disease, allowing easier and faster assessments and monitoring, and the accurate quantification of different parameters may open perspectives in establishing cut-offs for gait and balance characterization of Parkinson’s disease patients with peripheral neuropathy.
In conclusion, the results of this study suggest that clinicians and researchers should evaluate and consider peripheral neuropathy in the assessment of Parkinson’s disease, especially with regard to gait and balance difficulties as they increase Parkinson’s disease patient disability. Gait and balance complications of peripheral neuropathy may be partially addressed by optimizing L-DOPA therapy. Preventive peripheral neuropathy strategies and directed treatment, if effective, may decrease Parkinson’s disease patient disability. In addition, applying peripheral neuropathy-oriented physical therapy, technical aids, physical exercise and tactile or vibratory feedback techniques may prevent peripheral neuropathy progression and decrease patient disability. This work provides consistent evidence for the implementation of peripheral neuropathy assessment and treatment optimization aiming at individualized Parkinson’s disease patient care and quality of life improvement.
Supplementary Material
Acknowledgements
We thank Ana Luisa Sousa, Vanessa Oliveira, Diogo Costa, Sara Duarte, Sara Cavaco, Inês Ferreira, Marina Magalhães, Miguel Gago and João Felgueiras for their help in data collection, and Prof. Davide Cattaneo and Elke Warmerdam for the assistance with data analysis. We thank Lurdes Palhau for the support in the assessment facilities and Milene Pinto and Lina Silva for their help with the administrative work. We also thank the technicians from the Neuropathology lab for their assistance during all the lab experiments.
Contributor Information
Marta Francisca Corrà, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, 4050-313 Porto, Portugal; Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal; Institute for Research and Innovation in Health (i3s), University of Porto, 4200-135 Porto, Portugal.
Nuno Vila-Chã, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Ana Sardoeira, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Clint Hansen, Department of Neurology, Kiel University, 24118 Kiel, Germany.
Ana Paula Sousa, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Inês Reis, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Firmina Sambayeta, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Joana Damásio, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Margarida Calejo, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Andreas Schicketmueller, Institute for Medical Engineering and Research Campus STIMULATE, University of Magdeburg, 39106 Magdeburg, Germany; HASOMED GmbH, 39114 Magdeburg, Germany.
Inês Laranjinha, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Paula Salgado, Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Ricardo Taipa, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, 4050-313 Porto, Portugal; Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Rui Magalhães, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, 4050-313 Porto, Portugal; Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Manuel Correia, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, 4050-313 Porto, Portugal; Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal.
Walter Maetzler, Department of Neurology, Kiel University, 24118 Kiel, Germany.
Luís F Maia, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), University of Porto, 4050-313 Porto, Portugal; Centro Hospitalar Universitário do Porto (CHUPorto), 4099-001 Porto, Portugal; Institute for Research and Innovation in Health (i3s), University of Porto, 4200-135 Porto, Portugal.
Funding
This research was funded by Keep Control from the EU’s Horizon 2020 (H2020) research and innovation program under the Marie Sklodowska-Curie (MSCA-ITN-ETN), grant number 721577, and co-funded by the Portuguese Foundation for Science and Technology ‘Fundação para a Ciência e a Tecnologia' (M.F. Corrà 2020.08238.BD).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Hammarlund CS, Westergren A, Åström I, Edberg AK, Hagell P. The impact of living with Parkinson's disease: balancing within a web of needs and demands. Parkinsons Dis. 2018;2018:4598651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dennison AC, Noorigian JV, Robinson KM, et al. . Falling in Parkinson disease: identifying and prioritizing risk factors in recurrent fallers. Am J Phys Med Rehabil. 2007;86(8):621–632. [DOI] [PubMed] [Google Scholar]
- 3. Poewe W. Non-motor symptoms in Parkinson's disease. Eur J Neurol. 2008;15(s1):14–20. [DOI] [PubMed] [Google Scholar]
- 4. Takamatsu Y, Fujita M, Ho GJ, et al. . Motor and nonmotor symptoms of Parkinson’s disease: Antagonistic pleiotropy phenomena derived from α-synuclein evolvability? Parkinsons Dis. 2018;2018:5789424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Araújo DF, de Melo Neto AP, Oliveira ÍS, et al. . Small (autonomic) and large fiber neuropathy in Parkinson disease and parkinsonism. BMC Neurol. 2016;16:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mancini F, Comi C, Oggioni GD, et al. . Prevalence and features of peripheral neuropathy in Parkinson's disease patients under different therapeutic regimens. Parkinsonism Relat Disord. 2014;20(1):27–31. [DOI] [PubMed] [Google Scholar]
- 7. Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson's disease: A systematic review. J Neurol Sci. 2017;378:204–209. [DOI] [PubMed] [Google Scholar]
- 8. Barrell K, Smith AG. Peripheral neuropathy. Med Clin North Am. 2019;103(2):383–397. [DOI] [PubMed] [Google Scholar]
- 9. Hovaguimian A, Gibbons CH. Diagnosis and treatment of pain in small-fiber neuropathy. Curr Pain Headache Rep. 2011;15(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. England JD, Asbury AK. Peripheral neuropathy. Lancet. 2004;363(9427):2151–2161. [DOI] [PubMed] [Google Scholar]
- 11. Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16(11):934–944. [DOI] [PubMed] [Google Scholar]
- 12. Gasparotti R, Padua L, Briani C, Lauria G. New technologies for the assessment of neuropathies. Nat Rev Neurol. 2017;13(4):203–216. [DOI] [PubMed] [Google Scholar]
- 13. Devigili G, Rinaldo S, Lombardi R, et al. . Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devigili G, Tugnoli V, Penza P, et al. . The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbruzzese G, Pigullo S, Schenone A, et al. . Does parkin play a role in the peripheral nervous system? A family report. Mov Disord. 2004;19(8):978–981. [DOI] [PubMed] [Google Scholar]
- 16. Ohsawa Y, Kurokawa K, Sonoo M, et al. . Reduced amplitude of the sural nerve sensory action potential in PARK2 patients. Neurology. 2005;65(3):459–462. [DOI] [PubMed] [Google Scholar]
- 17. Toth C, Breithaupt K, Ge S, et al. . Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 2010;68(1):28–36. [DOI] [PubMed] [Google Scholar]
- 18. Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson's disease. Mov Disord. 2008;23(13):1850–1859. [DOI] [PubMed] [Google Scholar]
- 19. Ceravolo R, Cossu G, di Poggio MB, et al. . Neuropathy and levodopa in Parkinson's disease: evidence from a multicenter study. Mov Disord. 2013;28(10):1391–1397. [DOI] [PubMed] [Google Scholar]
- 20. Comi C, Magistrelli L, Oggioni GD, et al. . Peripheral nervous system involvement in Parkinson's disease: evidence and controversies. Parkinsonism Relat Disord. 2014;20(12):1329–1334. [DOI] [PubMed] [Google Scholar]
- 21. Misra UK, Kalita J, Nair PP. Diagnostic approach to peripheral neuropathy. Ann Indian Acad Neurol. 2008;11(2):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L. Levodopa-induced neuropathy: A systematic review. Mov Disord Clin Pract. 2019;6(2):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav. 2017;7(5):e00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doppler K, Ebert S, Uçeyler N, et al. . Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol. 2014;128(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth B, Schiro DB, Ohlsson B. Diseases which cause generalized peripheral neuropathy: a systematic review. Scand J Gastroenterol. 2021;56(9):1000–1010. [DOI] [PubMed] [Google Scholar]
- 26. Beaulieu ML, Müller M, Bohnen NI. Peripheral neuropathy is associated with more frequent falls in Parkinson's disease. Parkinsonism Relat Disord. 2018;54:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godinho C, Domingos J, Cunha G, et al. . A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson's disease. J Neuroeng Rehabil. 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corrà MF, Warmerdam E, Vila-Chã N, Maetzler W, Maia L. Wearable health technology to quantify the functional impact of peripheral neuropathy on mobility in Parkinson's disease: A systematic review. Sensors (Basel). 2020;20(22)6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 31. Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 32. Xiong Q, Lu B, Ye H, Wu X, Zhang T, Li Y. The diagnostic value of neuropathy symptom and change score, neuropathy impairment score and michigan neuropathy screening instrument for diabetic peripheral neuropathy. Eur Neurol. 2016;74(5–6):323–327. [DOI] [PubMed] [Google Scholar]
- 33. Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26(3):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complications. 2015;29(3):372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43(8):1508–1508. [DOI] [PubMed] [Google Scholar]
- 36. Dyck PJ, Dyck PJB, Grant IA, Fealey RD. Ten steps in characterizing and diagnosing patients with peripheral neuropathy. Neurology. 1996;47(1):10–17. [DOI] [PubMed] [Google Scholar]
- 37. Chong PST, Cros DP. Technology literature review: Quantitative sensory testing. Muscle & Nerve. 2004;29(5):734–747. [DOI] [PubMed] [Google Scholar]
- 38. Lauria G, Cornblath DR, Johansson O, et al. . EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12(10):747–758. [DOI] [PubMed] [Google Scholar]
- 39. Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. . A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333–338. [DOI] [PubMed] [Google Scholar]
- 40. Lauria G, Lombardi R. Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ. 2007;334(7604):1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hemminger A, Wills BK. Vitamin B6 Toxicity. In: StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 42. Tynes M, Hepprich M, Timper K. Regular intake of energy drinks and multivitamin supplements is associated with elevated plasma vitamin B6 levels in post-bariatric patients. Sci Re. 2021;11(1):17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. England JD, Gronseth GS, Franklin G, et al. . Evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Muscle Nerve. 2009;39(1):116–125. [DOI] [PubMed] [Google Scholar]
- 44. Micó-Amigo ME, Kingma I, Heinzel S, et al. . Potential markers of progression in idiopathic Parkinson’s disease derived from assessment of circular gait with a single body-fixed-sensor: A 5 year longitudinal study. Front Hum Neurosci. 2019;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pham MH, Elshehabi M, Haertner L, et al. . Validation of a step detection algorithm during straight walking and turning in patients with Parkinson's disease and older adults using an inertial measurement unit at the lower back. Front Neurol. 2017;8:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donath L, Faude O, Lichtenstein E, Nüesch C, Mündermann A. Validity and reliability of a portable gait analysis system for measuring spatiotemporal gait characteristics: comparison to an instrumented treadmill. J Neuroeng Rehabil. 2016;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mancini M, Salarian A, Carlson-Kuhta P, et al. . ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68(7):820–827. [DOI] [PubMed] [Google Scholar]
- 49. Mancini M, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Chiari L. Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Parkinsonism Relat Disord. 2011;17(7):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hansen C, Beckbauer M, Romijnders R, et al. . Reliability of IMU-derived static balance parameters in neurological diseases. Int J Environ Res Public Health. 2021;18(7)3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33. [DOI] [PubMed] [Google Scholar]
- 52. Podgorny PJ, Suchowersky O, Romanchuk KG, Feasby TE. Evidence for small fiber neuropathy in early Parkinson's disease. Parkinsonism Relat Disord. 2016;28:94–99. [DOI] [PubMed] [Google Scholar]
- 53. Szadejko K, Dziewiatowski K, Szabat K, et al. . Polyneuropathy in levodopa-treated Parkinson's patients. J Neurol Sci. 2016;371:36–41. [DOI] [PubMed] [Google Scholar]
- 54. Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology. 2011;77(22):1947–1950. [DOI] [PubMed] [Google Scholar]
- 55. Kass-Iliyya L, Javed S, Gosal D, et al. . Small fiber neuropathy in Parkinson's disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord. 2015;21(12):1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nolano M, Provitera V, Estraneo A, et al. . Sensory deficit in Parkinson's disease: Evidence of a cutaneous denervation. Brain. 2008;131(Pt 7):1903–1911. [DOI] [PubMed] [Google Scholar]
- 57. Novak P, Marya NB, Whren K, Bhawan J. Dermal sheet preparations in the evaluation of dermal innervation in Parkinson's disease and multiple system atrophy. J Cutan Pathol. 2009;36(3):296–301. [DOI] [PubMed] [Google Scholar]
- 58. Chentli F, Azzoug S, Mahgoun S. Diabetes mellitus in elderly. Indian J Endocrinol Metab. 2015;19(6):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maluf F C, Feder D, Alves de Siqueira Carvalho A. Analysis of the relationship between type II diabetes mellitus and Parkinson's disease: A systematic review. Parkinson's Dis. 2019;2019:4951379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jain A, Paranjape S. Prevalence of type 2 diabetes mellitus in elderly in a primary care facility: An ideal facility. Indian J Endocrinol Metab. 2013;17(Suppl 1):S318–S322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dietiker C, Kim S, Zhang Y, Christine CW. Characterization of vitamin b12 supplementation and correlation with clinical outcomes in a large longitudinal study of early Parkinson’s disease. JMD. 2019;12(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paul DA, Qureshi ARM, Rana AQ. Peripheral neuropathy in Parkinson’s disease. Neurol Sci. 2020;41(10):2691–2701. [DOI] [PubMed] [Google Scholar]
- 63. Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24(3):173–191. [DOI] [PubMed] [Google Scholar]
- 64. Lalli P, Chan A, Garven A, et al. . Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complications. 2013;27(3):248–254. [DOI] [PubMed] [Google Scholar]
- 65. Ling E, Lepow B, Zhou H, Enriquez A, Mullen A, Najafi B. The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes. Clin Biomech (Bristol, Avon). 2020;73:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Najafi B, Khan T, Fleischer A, Wrobel J. The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J Am Podiatr Med Assoc. 2013;103(3):165–173. [DOI] [PubMed] [Google Scholar]
- 67. Hazari A, Maiya AG, Shivashankara KN, et al. . 3D biomechanical analysis of foot in diabetes with and without peripheral neuropathy-a pilot study. Res J Pharm Biol Chem Sci. 2016;7(3):558–564. [Google Scholar]
- 68. Simmons RW, Richardson C, Pozos R. Postural stability of diabetic patients with and without cutaneous sensory deficit in the foot. Diabetes Res Clin Pract. 1997;36(3):153–160. [DOI] [PubMed] [Google Scholar]
- 69. Turcot K, Allet L, Golay A, Hoffmeyer P, Armand S. Postural strategies in diabetes patients with peripheral neuropathy determined using cross-correlation functions. Diabetes Technol Ther. 2012;14(5):403–410. [DOI] [PubMed] [Google Scholar]
- 70. Fino PC, Horak FB, El-Gohary M, et al. . Postural sway, falls, and self-reported neuropathy in aging female cancer survivors. Gait Posture. 2019;69:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82(1):167–177. [DOI] [PubMed] [Google Scholar]
- 72. Wuehr M, Schniepp R, Schlick C, et al. . Sensory loss and walking speed related factors for gait alterations in patients with peripheral neuropathy. Gait Posture. 2014;39(3):852–858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.