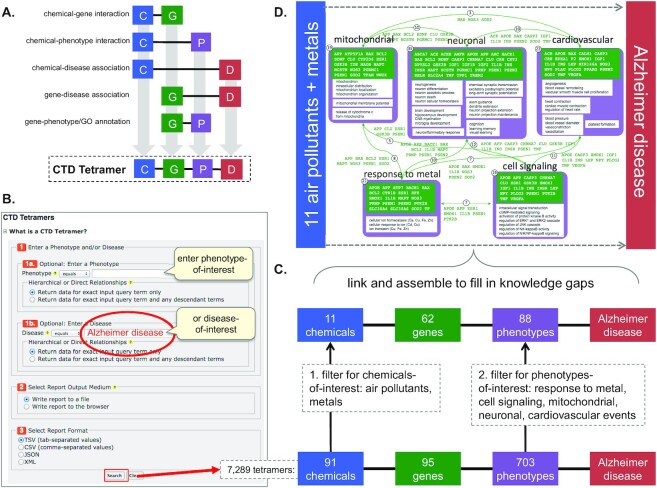
Figure 1.
New CTD Tetramer tool generates CGPD-tetramers that can help fill in knowledge gaps and construct potential molecular mechanistic pathways. (A) A CGPD-tetramer is a computationally generated information block composed of four units: an initiating chemical (C), an interacting gene (G), a modulated phenotype (P), and a disease (D) outcome. To generate a tetramer, five direct dyad evidence statements are integrated from CTD: C–G interaction, C–P interaction, C–D association, G–D association, and an imported G–P annotation, since GO biological process terms are the equivalent vocabulary for phenotypes in CTD (19). A tetramer will be generated only if all five direct dyad evidence statements currently exist in CTD. This computational process generates a selective, but supported, set of tetramers and, importantly, does not require a priori knowledge by the user. (B) The CTD Tetramer tool (http://ctdbase.org/tools/tetramerQuery.go) can be queried for any phenotype and/or environmental disease-of-interest to automatically generate all possible tetramers. (C) For Alzheimer disease, the tool generates 7289 tetramers, composed of 91 chemicals, 95 genes, and 703 phenotypes. This output can be manually sorted, surveyed and filtered to focus on any subset of chemicals-of-interest (here, air pollutants and metals) as well as phenotype clusters (e.g. response to metal, cell signaling, mitochondrial-related, neuron-related, and cardiovascular-related), resulting in a sub-set of 601 tetramers, composed of 11 chemicals, 62 genes and 88 unique phenotypes. (D) Users can manually assemble the tetramers by hand by linking them together using the shared genes (green boxes/text/arrows) that connect different phenotype clusters (purple boxes) to build a complex, interrelated map. This manual process, outlined in (21), fills knowledge gaps with potential molecular mechanistic steps (e.g. intermediate genes and phenotypes) that link air pollution/metal exposure to Alzheimer disease, producing a testable framework for experimental verification.