Figure 1: Separation of LotA deubiquitinase activities.
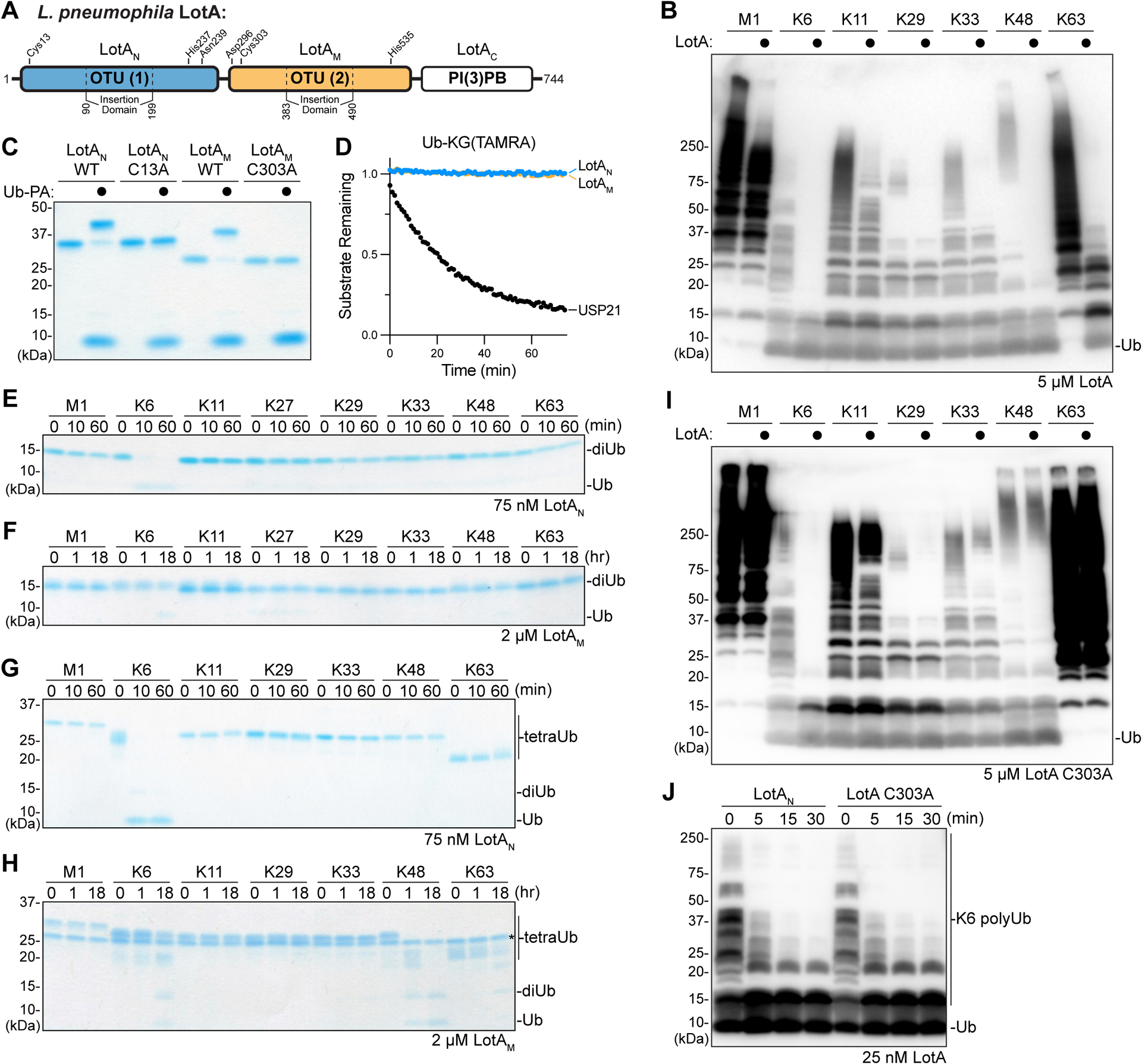
A. Domain architecture of L. pneuomophila LotA, with catalytic triad residues annotated for each OTU domain.
B. Homogeneous assemblies of seven polyUb linkage types were treated with a high concentration (5 μM) of full length LotA for 2 h before the reactions were quenched and visualized by anti-Ub Western blot.
C. Coomassie-stained SDS-PAGE gel showing purified constructs of LotAN (1–300) and LotAM (287–543), alongside their catalytically-inactive variants. Reactivity with the Ub-PA activity-based probe is assessed by a shift in mobility.
D. Ub-KG(TAMRA) cleavage assay monitored by fluorescence polarization. Activity was measured using 1 μM LotAN, 1 μM LotAM, or 100 nM USP21 as a control DUB.
E-F. Gel-based specificity analysis against all eight canonical diUb linkages. Reactions containing the indicated concentrations of LotAN (D) and LotAM (E) were sampled at the indicated timepoints, quenched, and resolved by SDS-PAGE with Coomassie staining.
G-H. Gel-based specificity analysis against seven tetraUb linkages. Reactions containing the indicated concentrations of LotAN (F) and LotAM (G) were sampled at the indicated timepoints, quenched, and resolved by SDS-PAGE with Coomassie staining. Note that some tetraUb chains migrate differently based on the linkage site, as observed previously38,45,46.
I. Homogeneous assemblies of seven polyUb linkage types were treated with a high concentration (5 μM) of full length LotA C303A for 2 h before the reactions were quenched and visualized by anti-Ub Western blot.
J. A homogeneous assembly of K6-linked polyUb was treated with either LotAN or the full length LotA C303A variant at 25 nM. Reaction samples were collected and visualized by anti-Ub Western blot.
See also Figure S1.
