Abstract
Background
Inclisiran, an siRNA administered twice-yearly, significantly reduced LDL cholesterol (LDL-C) in Phase III trials. Whether lowering LDL-C with inclisiran translates into a lower risk of cardiovascular (CV) events is not yet established.
Methods and results
Patient-level, pooled analysis of ORION-9, −10 and −11, included patients with heterozygous familial hypercholesterolaemia, atherosclerotic CV disease (ASCVD), or ASCVD risk equivalent on maximally tolerated statin-therapy, randomized 1:1 to receive 284 mg inclisiran or placebo on Days 1, 90, and 6-monthly thereafter for 18 months. Prespecified exploratory endpoint of major cardiovascular events (MACEs) included non-adjudicated CV death, cardiac arrest, non-fatal myocardial infarction (MI), and fatal and non-fatal stroke, evaluated as part of safety assessments using a standard Medical Dictionary for Regulatory Activities basket. Although not prespecified, total fatal and non-fatal MI, and stroke were also evaluated. Mean LDL-C at baseline was 2.88 mmol/L. At Day 90, the placebo-corrected percentage reduction in LDL-C with inclisiran was 50.6%, corresponding to an absolute reduction of 1.37 mmol/L (both P < 0.0001). Among 3655 patients over 18 months, 303 (8.3%) experienced MACE, including 74 (2.0%) fatal and non-fatal MIs, and 28 (0.8%) fatal and non-fatal strokes. Inclisiran significantly reduced composite MACE [OR (95% CI): 0.74 (0.58–0.94)], but not fatal and non-fatal MIs [OR (95% CI): 0.80 (0.50–1.27)] or fatal and non-fatal stroke [OR (95% CI): 0.86 (0.41–1.81)].
Conclusion
This analysis offers early insights into the potential CV benefits of lowering LDL-C with inclisiran and suggests potential benefits for MACE reduction. These findings await confirmation in the larger CV outcomes trials of longer duration.
Keywords: Inclisiran, LDL-C, Major adverse cardiovascular events, Atherosclerotic cardiovascular disease
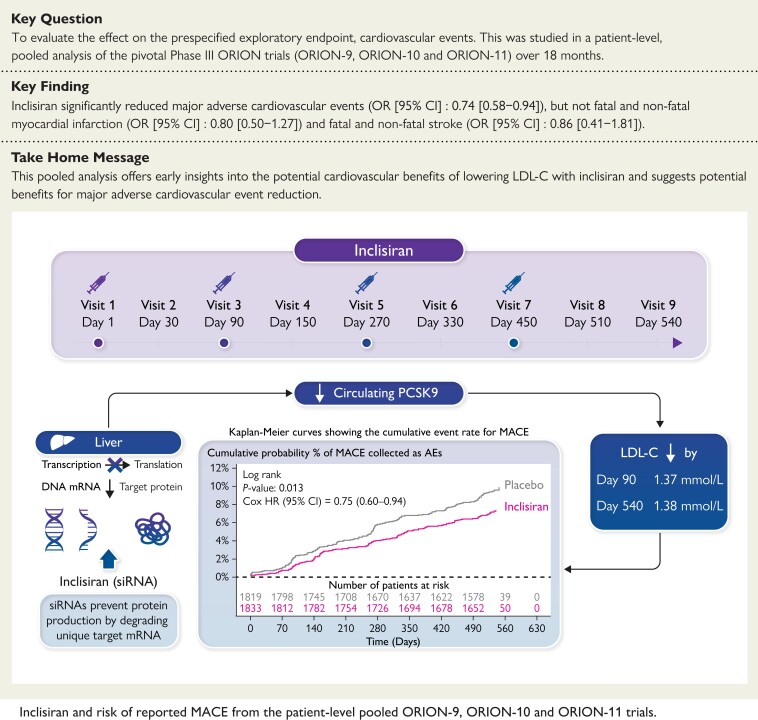
Exploratory findings from a patient-level, pooled analysis of the pivotal Phase III ORION trials (−9, −10, and −11) suggest that treatment with a 6 monthly dosing schedule of inclisiran (after the initial and three-month doses) over 18 months is associated with reductions in the composite endpoint of major cardiovascular events.
Structured Graphical Abstract
Structured Graphical Abstract.
AE, adverse event; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; mRNA, messenger ribonucleic acid; OR, odds ratio; PCSK9, proprotein convertase subtilisin/kexin type 9; siRNA, small interfering ribonucleic acid.
See the editorial comment for this article ‘Cholesterol lowering with inclisiran: a new chapter in the PCSK9 story book’, by Raul D. Santos and Viviane Z. Rocha, https://doi.org/10.1093/eurheartj/ehac656.
Introduction
Mendelian randomization studies assessing therapeutic targets for pharmacological interventions suggest that lowering LDL cholesterol (LDL-C) through pathways related to 3-hydroxy-3-methylglutaryl–coenzyme (HMGCo-A) reductase (statin target), Niemann-Pick C1-like 1 (NCP1L1; ezetimibe target), and proprotein convertase subtilisin/kexin type 9 (PCSK9; PCSK9 monoclonal antibody target) are associated with similar reductions in the risk of cardiovascular (CV) events when standardized for absolute difference in LDL-C.1,2 This suggests that the magnitude of the reduction in LDL-C determines benefit rather than the mechanism through which this is achieved.2 Mendelian randomization studies also suggest that greater reductions in LDL-C can be achieved through the combination of either pathways related to HMGCo-A reductase and NCP1L-1 or HMGCo-A reductase and PCSK9, with greater benefit because of greater lifelong absolute reductions in LDL-C achieved by multiple pathways.1–3 These observations are mirrored by trials of therapeutic LDL-C lowering, where the addition of ezetimibe4 or PCSK9 inhibitors5,6 to statins results in further reductions in CV events, related to the absolute reduction in LDL-C and duration of exposure.2
Clinical trials have demonstrated benefits from achieving sequentially lower LDL-C levels among those at highest risk of CV events, resulting in guidelines progressively lowering LDL-C goals for intensification of lipid-lowering therapy in successive iterations of practice guidelines.7 Achieving these currently recommended, lower LDL-C goals/thresholds often necessitates combinations of lipid-lowering therapies for the majority of patients with atherosclerotic CV disease (ASCVD).8 Monoclonal antibodies (mAbs) targeting circulating PCSK9 are potent therapies that lower LDL-C by 50–70%.9 As the majority of circulating PCSK9 is derived from the liver, preventing hepatic production of PCSK9 offers an alternative approach to lower circulating, free PCSK9, and thus lower LDL-C. Inclisiran is a small interfering ribonucleic acid (siRNA) that prevents hepatic PCSK9 production.10 Inclisiran, in combination with diet and maximally tolerated statins, has been approved for cholesterol-lowering in patients with primary hypercholesterolaemia or mixed dyslipidaemia by the European Medicines Agency and treatment of patients with heterozygous familial hypercholesterolaemia (HeFH) or clinical ASCVD by the United States Food and Drug Administration.11,12 Three Phase III lipid-lowering trials including patients at high risk of CV events have shown that inclisiran reduces circulating PCSK9 and LDL-C.13,14 Whether lowering LDL-C with inclisiran reduces the risk of CV events has not yet been established and is being evaluated in dedicated ongoing CV outcomes trials ORION-4 (NCT03705234) and VICTORION-2 Prevent (NCT05030428). To provide early insights into the potential for this therapeutic approach, we pooled individual participant data from the Phase III lipid-lowering trials, each with 18 months of follow-up and together comprised of 3655 individuals, to assess the relationship between inclisiran treatment or placebo on the risk of CV events.
Methods
Study design and population
In each of the three pivotal Phase III placebo-controlled trials that evaluated the lipid-lowering efficacy of inclisiran, major adverse cardiovascular events (MACE) comprised a prespecified exploratory endpoint. The present prespecified analysis encompasses pooled data from the three trials. The study design, methods, and results for these trials have been described in detail previously.13,14 The same academic steering committee provided trial oversight for the three trials, with a single common data safety monitoring committee and identical study designs facilitating data pooling. Patients were randomized 1:1 in each trial to receive either inclisiran sodium 300 mg (equivalent to inclisiran 284 mg) or placebo subcutaneously, administered on Day 1, Day 90, and 6-monthly thereafter for a total duration of 18 months. Clinic visits were scheduled on Days 30, 90, 150, 270, 330, 450, 510, and 540.
Each trial included patients at high risk of CV events who, despite receiving maximally tolerated doses of statins, had elevated LDL-C levels. The populations studied included patients with HeFH (ORION-9), ASCVD (ORION-10, ORION-11), and high-risk, primary prevention patients, henceforth referred to as ASCVD risk equivalent (ORION-11). The latter consisted of individuals with no known prior history of ASCVD, but who had either type 2 diabetes mellitus or HeFH, or a predicted 10-year risk of >20% using Framingham risk score for CV disease or equivalent.
The primary population for the efficacy analyses was the intention-to-treat (ITT) population, which included all randomized patients. The primary population for the safety analyses (including the CV assessments) comprised of all patients who received at least one dose of inclisiran or placebo (see Supplementary material online, Table S1).
LDL-C levels
Changes in LDL-C from baseline to each assessment visit were measured up to Day 540 and have been described previously.15 The placebo-corrected percentage and absolute changes in LDL-C were assessed at the first (Day 90) and last (Day 540) visits for each trial and subsequently pooled without censoring for CV events that may have occurred prior to that visit, for the intention-to-treat population.
Cardiovascular events
None of the trials included in the present analysis were CV outcomes trials or had a formal endpoint adjudication committee. Therefore, the present analysis used CV events that were reported as adverse events (AEs) by a study physician and entered into the safety population database. Relevant AEs [MACE, myocardial infarction (MI) and stroke] were identified using standard nomenclature from the Medical Dictionary for Regulatory Activities (MedDRA) version v20.1 (see Supplementary material online, Table S2). A basket of MedDRA-defined CV terms was used to define the prespecified, non-adjudicated, exploratory endpoint of MACE (defined as per protocol) as a composite of cardiac death, cardiac arrest, non-fatal MI, and fatal and non-fatal stroke. In addition, two non-prespecified endpoints, fatal and non-fatal MI, and fatal and non-fatal stroke, were also evaluated using a modified definition (definitions of MACE, fatal and non-fatal MI and stroke can be found in the Supplementary material online, Table S2).
Statistical analysis
Evaluation of differences in LDL-C levels was based on the ITT population, which was prespecified for the evaluation of lipid-lowering efficacy (N = 3660; Supplementary material online, Table S1). Treatment comparisons assessing differences in LDL-C were performed using a mixed model for repeated measures. The restricted maximum likelihood estimation approach was used with the covariance structure set as unstructured. SAS (Statistical Analysis Software) v9.4 was used for the analysis. The analysis of MACE was based on the safety population, which consisted of all patients who received study medication (n = 3655; Supplementary material online, Table S1). The data were pooled and analysed using Cox regression methods as well as Kaplan–Meier survival analysis. Hazard ratios (HRs) were derived from Cox regression methods. The Kaplan–Meier method was used to estimate cumulative event rates, and log-rank tests were used to compare time-to-first event. To account for differences in the contribution of the number of events from different studies, and to allow for weighting of events for outcomes of interest, we first used the number of patients with events (counts) to calculate an odds ratio (OR) for each outcome within each of the three trials. Data were then meta-analysed based on the number of patients experiencing MACE in each trial. The meta-analysis was performed using the Mantel-Haenszel approach with the Peto method for pooling the studies, using the study as a fixed effect in the model.16 The Peto method for pooling studies was used to ensure that data from all three ORION studies were included in the meta-analysis, even for cases where no patients had an event in a study. ORs and corresponding 95% confidence intervals (CIs) are provided for the meta-analysis as estimates of overall effect. The meta-analysis was performed using R version 3.5.3. The ‘metafor’ libraries were used.
Results
Baseline characteristics
The ITT population used for efficacy analyses consisted of 3660 patients, of whom 1833 were randomly assigned to receive inclisiran and 1827 to receive placebo. The mean (SD) age of the total population was 64.0 (9.9) years, with approximately 32.5% (n = 1190) women. While the majority had ASCVD, 15.1% (n = 553) had ASCVD risk equivalent. Among patients with ASCVD, approximately 89.5% (n = 2782) had a prior or current history of coronary heart disease, 16.4% (n = 511) had cerebrovascular disease, 10.5% (n = 325) had peripheral artery disease and 37.4% (n = 1162) had diabetes. Among patients with ASCVD risk equivalent, 28.2% (n = 156) had diabetes mellitus, 68.4% (n = 378) had familial hypercholesterolaemia and 20.6% (n = 114) had an estimated 10-year risk of a CV event >20%. CV risk factors and comorbidities were common in the overall study population; 44.9% (n = 1642) had chronic kidney disease (defined as an estimated glomerular filtration rate of ≥15 to <90 mL/min/1.73 m2 at baseline), 12.0% (n = 440) had a prior history of congestive heart failure, 79.8% (n = 2919) had a history of hypertension and 15.9% (n = 582) were current smokers. Overall, 94.0% (n = 3441) of patients were receiving statins or other lipid-lowering therapies at baseline, of whom 91.8% (n = 3361) were on statins, including 73.8% on high-intensity statins and 14.2% on ezetimibe (Table 1).
Table 1.
Baseline demographic and clinical characteristics (ITT population)
| Characteristics | ORION-9, ORION-10 and ORION-11 pooled15 | ORION-913 | ORION-1014 | ORION-1114 | ||||
|---|---|---|---|---|---|---|---|---|
| Inclisiran | Placebo | Inclisiran | Placebo | Inclisiran | Placebo | Inclisiran | Placebo | |
| n = 1833 | n = 1827 | n = 242 | n = 240 | n = 781 | n = 780 | n = 810 | n = 807 | |
| Age, years, mean (SD) | 64.1 (9.98) | 63.9 (9.87) | 56 (47–63)a | 56 (46–64)a | 66.4 (8.9) | 65.7 (8.9) | 64.8 (8.3) | 64.8 (8.7) |
| Male, n (%) | 1226 (66.9) | 1244 (68.1) | 112 (46.3) | 115 (47.9) | 535 (68.5) | 548 (70.3) | 579 (71.5) | 581 (72.0) |
| ASCVD, n (%) | 1552 (84.7) | 1555 (85.1) | 59 (24.4) | 73 (30.4) | 781 (100) | 780 (100) | 712 (87.9) | 702 (87.0) |
| Prior MI, n (%) | 831 (45.3) | 863 (47.2) | 22 (9.1) | 29 (12.1) | 375 (48.0) | 410 (52.6) | 434 (53.6) | 424 (52.5) |
| CHF, n (%) | 213 (11.6) | 227 (12.4) | 4 (1.7) | 9 (3.8) | 100 (12.8) | 116 (14.9) | 109 (13.5) | 102 (12.6) |
| CV risk factors, n (%) | ||||||||
| ȃSmoking (current) | 311 (17.0) | 271 (14.8) | 28 (11.6) | 28 (11.7) | 123 (15.7) | 111 (14.2) | 160 (19.8) | 132 (16.4) |
| ȃHypertension | 1456 (79.4) | 1463 (80.1) | 102 (42.1) | 101 (42.1) | 714 (91.4) | 701 (89.9) | 640 (79.0) | 661 (81.9) |
| ȃDiabetes | 687 (37.5) | 631 (34.5) | 20 (8.3) | 28 (11.7) | 371 (47.5) | 331 (42.4) | 296 (36.5) | 272 (33.7) |
| Lipid-lowering therapy, n (%) | ||||||||
| ȃStatins | 1686 (92.0) | 1675 (91.7) | 219 (90.5) | 217 (90.4) | 701 (89.8) | 692 (88.7) | 766 (94.6) | 766 (94.9) |
| ȃHigh-intensity statins | 1356 (74.0) | 1345 (73.6) | 185 (76.4) | 171 (71.3) | 538 (68.9) | 546 (70.0) | 633 (78.1) | 628 (77.8) |
| ȃEzetimibe | 251 (13.7) | 270 (14.8) | 119 (49.2) | 134 (55.8) | 80 (10.2) | 74 (9.5) | 52 (6.4) | 62 (7.7) |
| Anti-platelet therapy, n (%) | ||||||||
| ȃASA | 1309 (71.4) | 1286 (70.4) | 84 (34.7) | 89 (37.1) | 614 (78.6) | 614 (78.7) | 611 (75.4) | 583 (72.2) |
| ȃP2Y12ib | 442 (24.1) | 499 (27.3) | 12 (5.0) | 13 (5.4) | 283 (36.2) | 312 (40.0) | 147 (18.1) | 174 (21.6) |
| Lipid measures, mmol/L, mean (SD) | ||||||||
| ȃLDL-C | 2.89 (1.16) | 2.87 (1.13) | 3.92 (1.30) | 4.00 (1.50) | 2.70 (1.02) | 2.71 (0.96) | 2.77 (1.08) | 2.68 (0.94) |
| ȃHDL-C | 1.26 (0.39) | 1.24 (0.36) | 1.33 (0.39) | 1.31 (0.34) | 1.20 (0.37) | 1.19 (0.37) | 1.29 (0.40) | 1.27 (0.36) |
| HbA1c (%), median (IQR) | 5.9 (5.6, 6.5) | 5.9 (5.6, 6.5) | 5.6 (5.4, 5.9) | 5.5 (5.4, 5.8) | 6.1 (5.6, 7.0) | 6.0 (5.6, 6.9) | 5.9 (5.6, 6.5) | 5.9 (5.6, 6.4) |
Age, years, mean (IQR).
P2Y12i includes clopidogrel, prasugrel, and ticagrelor.
ASA, acetylsalicylic acid; ASCVD, atherosclerotic cardiovascular disease; CHF, congestive heart failure; CV, cardiovascular; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; ITT, intention-to-treat; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; n, number of patients; P2Y12i, purinergic signalling receptor Y12 inhibitor; SD, standard deviation.
Change in LDL-C levels
At baseline, the mean LDL-C was 2.88 mmol/L (111.4 mg/dL) in the pooled analysis. At Day 90, the placebo-corrected percentage and absolute reductions in LDL-C levels from baseline were 50.6% [95% CI (−52.3 to −49.0); P < 0.0001] and 1.37 mmol/L [95% CI (−1.42 to −1.33); P < 0.0001], respectively.
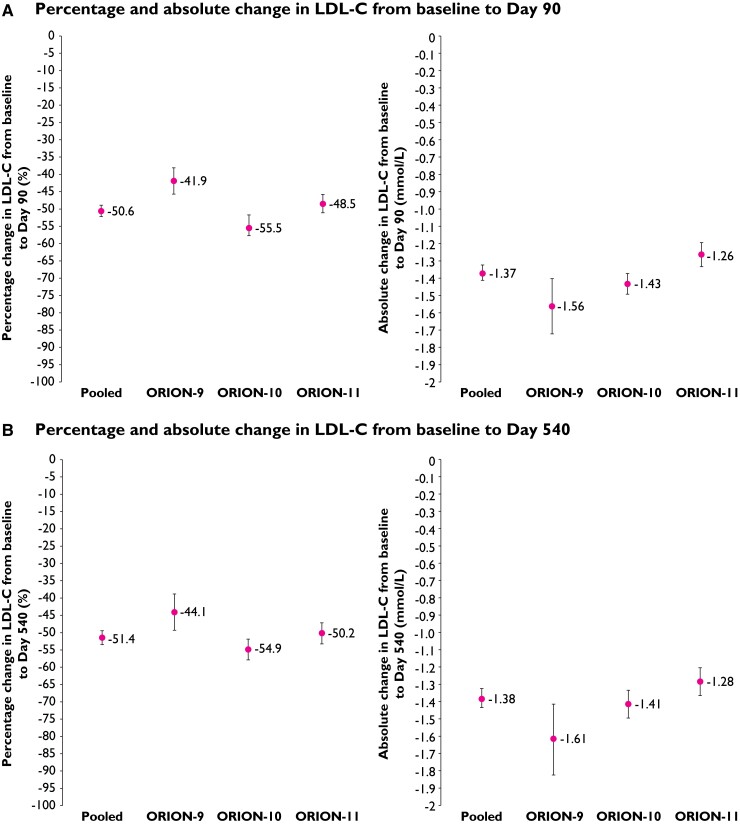
Figure 1 shows the percentage and absolute changes in LDL-C levels in the pooled analysis and individual trials. Differences in LDL-C were sustained through the end of the study visit. At Day 540, the placebo-corrected percentage reduction in LDL-C from baseline with inclisiran was 51.4% [95% CI (−53.4 to −49.4); P < 0.0001] with an absolute reduction of 1.38 mmol/L [95% CI (−1.44 to −1.33); P < 0.0001] in the pooled analysis (Figure 1).
Figure 1.
Mean placebo-corrected percentage and absolute changes in LDL-C from baseline to (intention-to-treat population): (A) Day 90 and (B) Day 540. Values shown are mean treatment difference (inclisiran vs. placebo) and error bars represent the 95% confidence interval (mixed model repeated measures analysis); P < 0.0001 for all.
Risk of reported major adverse cardiovascular event, myocardial infarction, stroke, and death
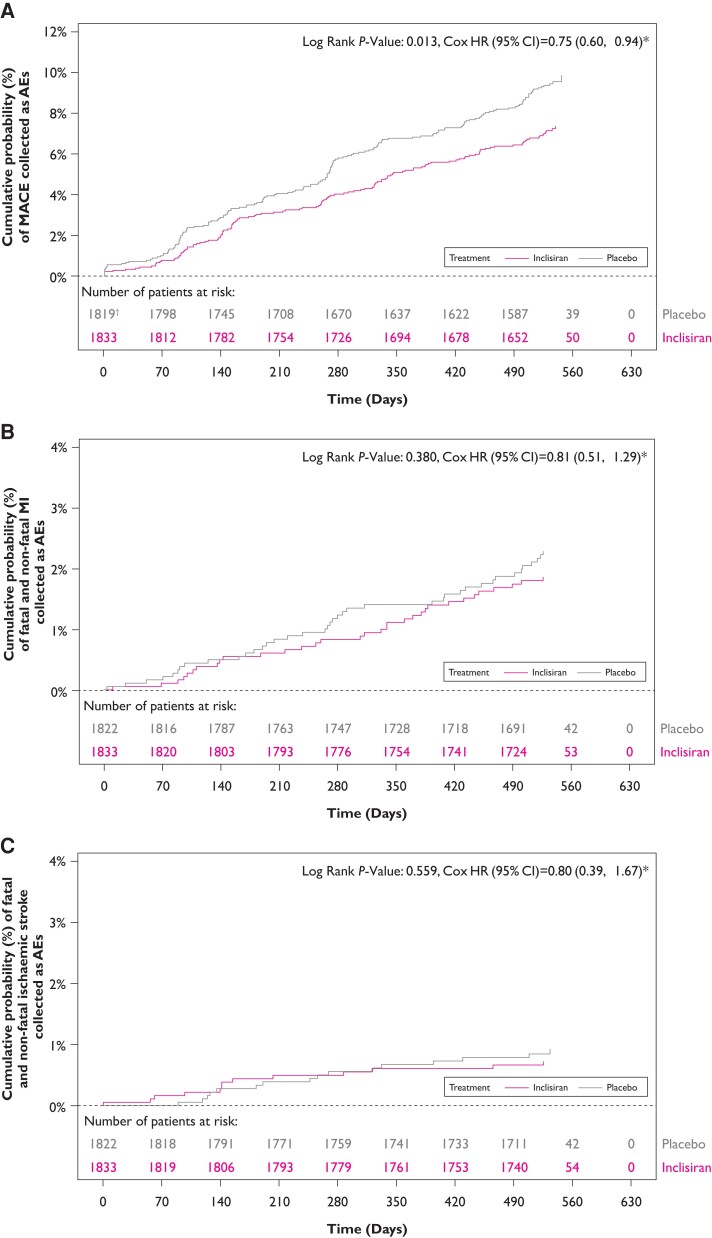
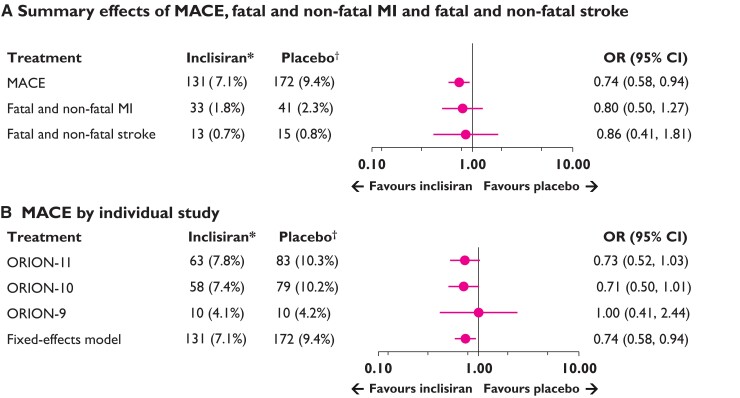
The safety population included a total of 3655 patients (inclisiran [n = 1833]; placebo [n = 1822]). Incidence of reported MACE, fatal and non-fatal MI, and fatal and non-fatal stroke are provided in Table 2. Overall, fewer patients who received inclisiran experienced MACE compared with placebo (131 vs. 172), reflecting differences in annualized rates of 5.35 per 100 person-years vs. 7.71 per 100 person-years, respectively. Similarly, there were fewer fatal and non-fatal MI (33 vs. 41), and fatal and non-fatal stroke (13 vs. 15) events among those allocated inclisiran as compared with those receiving placebo. At Day 540, Kaplan–Meier estimate rates for MACE, fatal and non-fatal MI, and fatal and non-fatal stroke were 7.4% vs. 9.5%, 1.9% vs. 2.3% and 0.7% vs. 0.9% in the inclisiran and placebo treatment groups, respectively (Figure 2). Using the Cox regression, the HR for inclisiran vs. placebo was 0.75 (95% CI 0.60–0.94) for MACE, 0.81 (95% CI 0.51–1.29) for fatal and non-fatal MI, and 0.80 (95% CI 0.39–1.67) for fatal and non-fatal stroke (Figure 2). Synthesis of data from each trial using inverse weighting and combined using a fixed effects meta-analysis are shown in Figure 3. OR for risk of each endpoint were consistent with time-to-event analyses for inclisiran vs. placebo for MACE [OR (95% CI): 0.74 (0.58–0.94)], fatal and non-fatal MI [OR (95% CI): 0.80 (0.50–1.27)], and fatal and non-fatal stroke [OR (95% CI): 0.86 (0.41–1.81)]. Finally, regarding cause-specific mortality, there were 17 vs. 15 CV deaths and 10 vs. 12 non-CV deaths in inclisiran and placebo groups, respectively, which reflect an overall 27 deaths from any cause in the inclisiran group and 27 deaths in the placebo group.
Table 2.
Cardiovascular events in the ORION-9, ORION-10, and ORION-11 trials (Safety population)a
| Variable | Trials | Inclisiranb n (%) |
Events | Placebob n (%) |
Events | Odds ratio (95% CI) |
|---|---|---|---|---|---|---|
| MACE | Pooled ORION-9, ORION-10 and ORION-11 | 131 (7.1) | 141 | 172 (9.4) | 201 | 0.74 (0.58–0.94) |
| ORION-9b | 10 (4.1) | 10 | 10 (4.2) | 11 | 1.00 (0.41–2.44) | |
| ORION-10b | 58 (7.4) | 66 | 79 (10.2) | 90 | 0.71 (0.50–1.01) | |
| ORION-11b | 63 (7.8) | 65 | 83 (10.3) | 100 | 0.73(0.52–1.03) | |
| Fatal and non-fatal MI | Pooled ORION-9, ORION-10 and ORION-11 | 33 (1.8) | 34 | 41 (2.3) | 45 | 0.80 (0.50–1.27) |
| Fatal and non-fatal stroke | Pooled ORION-9, ORION-10 and ORION-11 | 13 (0.7) | 14 | 15 (0.8) | 16 | 0.86 (0.41–1.81) |
Values shown are n (%) unless otherwise indicated. Definitions of MACE, fatal and non-fatal MI, and fatal and non-fatal stroke are provided in the Supplementary material online, Table S2.
aThe safety population included all the patients who received at least one dose of inclisiran or placebo.bNumber of patients in the inclisiran vs. placebo arms in the pooled and the individual ORION-9, ORION-10 and ORION-11 were 1833 vs. 1822, 241 vs. 240, 781 vs. 778, and 811 vs. 804, respectively.
CI, confidence interval; MACE, major adverse cardiovascular event; MI, myocardial infarction.
Figure 2.
Kaplan–Meier curves depicting the cumulative event rate (safety population): (A) major adverse cardiovascular event, (B) fatal and non-fatal myocardial infarction, and (C) fatal and non-fatal stroke. *The hazard ratio and 95% confidence interval are from a Cox model with treatment and study ID as factors. †The major adverse cardiovascular event count includes both non treatment-emergent adverse events and treatment-emergent adverse events. In the placebo arm, three non treatment-emergent adverse events occurred on or after the Screening date, within seven days of first dose, but prior to first dose. To avoid a ‘negative time-to-first event’ for these three patients, these events were not included in the time-to-first event analysis; instead, these patients were treated as missing.
Figure 3.
Fixed effects meta-analysis of the safety population: (A) major adverse cardiovascular event, fatal and non-fatal myocardial infarction and fatal and non-fatal stroke (pooled data from ORION-9, ORION-10 and ORION-11), and (B) major adverse cardiovascular event by individual study. *Inclisiran, n = 1833; †Placebo, n = 1822. The meta-analysis used the Peto method16 for pooling studies to ensure that patients enrolled in ORION-9 were included in the analysis, although there were no reported stroke events in the ORION-9 trial. The model used for the meta-analysis included study as a fixed effect. The major adverse cardiovascular event count included treatment emergent adverse events and non- treatment emergent adverse events. Fatal and non-fatal stroke counts included only serious adverse events. Definitions of major adverse cardiovascular event, fatal and non-fatal myocardial infarction, and fatal and non-fatal stroke are provided in the Supplementary material online, Table S2. Test of heterogeneity and I2for major adverse cardiovascular event or adverse cardiovascular event, Q = 0.48 P = 0.79; I2 = 0.00%.
Other safety parameters
Inclisiran was well-tolerated, except for the clinically relevant AEs at the injection site, which were more frequent in patients treated with inclisiran compared with placebo [n = 91 (5%) vs. n = 12 (0.7%); risk ratio (95% CI), 7.54 (4.14–13.71)]. Notably, the clinically relevant AEs at the injection site in the treatment arms were mostly mild [n = 67 (3.7%) vs. 11 (0.6%); RR (95% CI), 6.05 (3.21–11.42)], few moderate [n = 24 (1.3%) vs. n = 1(0.1%); RR (95% CI), 23.86 (3.23–176.15)] and none were severe or persistent. Additionally, a modest excess of mild–to–moderate bronchitis was reported in patients treated with inclisiran vs. placebo [n = 78 (4.3%) vs. n = 50 (2.7%); RR (95% CI), 1.55 (1.09–2.20)]. Laboratory evaluations that included liver and renal function tests, creatine kinase and platelet counts were similar between the two treatment groups.15
Discussion
This prespecified, patient-level, pooled analysis, including 3655 (safety population) high-risk patients (reflecting 2653 patient-years of follow-up with inclisiran), reported 303 patients with MACE, including 74 patients with fatal and non-fatal MI, and 28 patients with fatal and non-fatal stroke. The data presented here showed that the addition of inclisiran to background lipid-lowering therapies was associated with a 26% lower probability of MACE [OR (95% CI): 0.74 (0.58, 0.94)] and favourable trends towards a lower risk of fatal and non-fatal MI compared with placebo (Structured Graphical Abstract ). Strokes occurred infrequently in the trials, though were numerically less in the inclisiran treatment arm. Observed differences in CV outcomes with inclisiran treatment were achieved in a pooled cohort with a baseline LDL-C of approximately 2.88 mmol/L (111.4 mg/dL), despite background statin use in 91.8% of patients and ezetimibe in 14.2%. In accordance with the prespecified statistical analysis plan, the comparison of inclisiran with placebo for lipid-lowering utilized the ITT population, while the comparison for MACE utilized the safety population. These two populations differed by only 5 patients. Overall, the reported rate of AEs with inclisiran was similar to that of placebo, apart from bronchitis and clinically relevant AEs at the injection site, which were more frequent with inclisiran but were generally mild, and none were persistent. These safety and tolerability findings are consistent with those previously reported in the individual studies and in a prior detailed pooled safety analysis.13–15
Meta-regression analyses of large CV outcomes trials with a long follow-up duration suggest that each one mmol/L (38.7 mg/dL) reduction in LDL-C levels leads to a 23% reduction in the rate of MACE, irrespective of the therapy used to lower LDL-C.17 Therefore, after diet and lifestyle, lowering LDL-C through pharmacological approaches is an important strategy for the prevention of ASCVD and CV events, in both high-risk primary and secondary prevention settings.2,18 However, the CV benefits derived from a one mmol/L lowering of LDL-C levels in the first year of statin treatment are approximately half than those observed in later years.2 CV outcomes trials with PCSK9 mAbs demonstrate similar findings with greater benefits observed in the second and subsequent years compared with the first year of treatment.6,19 Notably, the relative benefits derived each year with PCSK9 mAb treatment is statistically indistinguishable from those observed with statins when standardized for LDL-C difference.20 In the SPIRE-2, FOURIER, and Cholesterol Treatment Trialists Collaboration (CTT) studies, the relative risk reductions per 1 mmol/L LDL-C lowering in year one of treatment were 14% [HR (95% CI): 0.86 (0.75, 0.98)], 13% [HR (95% CI): 0.87 (0.79, 0.97)] and 12% [HR (95% CI): 0.88 (0.84, 0.93)], respectively.20 The narrower CI reported with statins likely reflects the greater precision around the point estimate, derived from the accumulation of a larger number of events in the CTT meta-analysis vs. individual PCSK9 mAb trials. In year 2 of treatment in the FOURIER trial and CTT meta-analysis, the CV risk reductions per 1 mmol/L LDL-C decrease were 22% [HR (95% CI): 0.78 (0.71, 0.86)] and 23% [HR (95% CI): 0.77 (0.73, 0.82)], respectively, providing a combined reduction in risk over 2 years of 17% [HR (95% CI): 0.83 (0.70, 0.90)] and 17% [HR (95% CI): 0.83 (0.80, 0.86)], respectively. Although the absolute reduction in LDL-C in the present study was 1.38 mmol/L (53.5 mg/dL), the exposure time was only 18 months. Nevertheless, it is encouraging that the observed point estimate of 0.74 (albeit with wide CIs) in reported MACE is broadly consistent with observations from these larger studies. The benefit of lipid-lowering therapy with PCSK9 inhibitors on CV events, in particular, any impact on death, requires greater long-term absolute reduction of LDL-C levels, as suggested by the recent open label extension of the FOURIER trial and the randomized findings from the longer-term ODYSSEY OUTCOMES trial.21,22 In the analysis presented here, the full effect on CV events is limited by the short time of exposure to LDL-C lowering with inclisiran.
There are limitations of the present analysis that merit consideration. First, none of the individual component trials or the three trials in aggregate were powered to provide a reliable estimate of the CV benefits of inclisiran. Nonetheless, the number of MACE in the current analysis (∼300) is substantially greater than that in comparable analyses of the Phase III lipid-lowering trials with PCSK9 mAbs, that preceded the outcomes trials with those agents.5,23 Accurate determination of the effects of a lipid-lowering therapy on CV events requires studies that are larger and of longer duration than the current ones, with an accrual of several-fold more events. A second limitation is that ascertainment of MACE was based on investigator-reported AE-reporting rather than blinded adjudication by a panel of experts. Recent analyses of large CV outcomes trials have shown that the adjudicated rate of MACE is somewhat lower than the investigator-reported rate, but treatment hazard ratios differ minimally.24,25 Finally, whilst prespecified, it should be recognized that this analysis of MedDRA-defined CV terms was an exploratory analysis of the ORION-9, ORION-10 and ORION-11 trials.
With these caveats, the point estimates for MACE and specific CV events in the current analysis should be considered hypothesis-generating. The definitive test of the hypothesis that twice-yearly administration of inclisiran reduces MACE in patients with clinical ASCVD or high CV risk awaits results of the ongoing ORION-4 and VICTORION-2 Prevent trials. Each of these trials will enrol approximately 15 000 patients with clinical ASCVD and elevated LDL-C despite statin therapy, with an expected median follow-up of approximately 5 years and accrual of approximately 1600 to 1700 blindly adjudicated events.
In conclusion, this pooled analysis of high-risk patients with elevated LDL-C offers early insights into the potential CV benefits of inclisiran and suggests that this approach may provide CV benefits. These potential benefits of inclisiran should be considered in the context of a generally good tolerability profile, including a modest excess of mild-to-moderate AEs at the injection site and bronchitis compared with placebo.
Supplementary Material
Acknowledgements
The authors thank all the investigators, trial site staff, and patient volunteers who participated in the trials. Analysis was carried out by Summit Analytical, Denver, CO, USA, with funding from Novartis Pharma AG, Basel, Switzerland. The authors thank Vennila Dharman, MBBS (Novartis Healthcare Pvt. Ltd. India) and Aisling Towell, PhD (Novartis Ireland Ltd.) for providing medical writing support in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and Klaus Molle, PhD (Novartis Pharma AG) for critical review of the manuscript and editorial guidance. Prof Ray receives support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre; his institution (Imperial College London) receives support from the NIHR Applied Research Collaboration Northwest London.
Contributor Information
Kausik K Ray, Imperial Centre for Cardiovascular Disease Prevention, Department of Primary Care and Public Health, Imperial College, London, UK.
Frederick J Raal, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
David G Kallend, DalCor Pharmaceuticals, Montreal, Quebec, Canada; LIB Therapeutics, Cincinnati, OH, USA.
Mark J Jaros, Summit Analytical, Denver, CO, USA.
Wolfgang Koenig, German Heart Centre, Technical University Munich, DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany; Institute of Epidemiology and Medical Biometry, University of Ulm, Ulm, Germany.
Lawrence A Leiter, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, Canada.
Ulf Landmesser, Department of Cardiology, Charité-University Medicine Berlin, Berlin Institute of Health (BIH), DZHK, Partner Site, Berlin, Germany.
Gregory G Schwartz, Division of Cardiology, University of Colorado School of Medicine, Aurora, CO, USA.
David Lawrence, Novartis Pharma AG, Basel, Switzerland.
Andrew Friedman, Population Health Partners, Short Hills, NJ, USA.
Lorena Garcia Conde, Novartis Pharma AG, Basel, Switzerland.
R Scott Wright, Division of Preventive Cardiology and the Department of Cardiology, Mayo Clinic, Rochester, MN, USA.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Novartis Pharma AG, Basel, Switzerland.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, et al. . Mendelian Randomization study of ACLY and cardiovascular disease. N Engl J Med 2019;380:1033–1042. 10.1056/NEJMoa1806747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. . Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J 2017;38:2459–2472. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. . Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–2153. 10.1056/NEJMoa1604304 [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. 10.1056/NEJMoa1500858 [DOI] [PubMed] [Google Scholar]
- 6. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 7. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 8. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, et al. . EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–1289. 10.1093/eurjpc/zwaa047 [DOI] [PubMed] [Google Scholar]
- 9. Najam O, Lambert G, Ray KK. The past, present and future of lipid lowering therapy. Clin Lipidol 2015;10:481–498. doi: 18 Jan 2017. [Google Scholar]
- 10. Macchi C, Sirtori CR, Corsini A, Santos RD, Watts GF, Ruscica M. A new Dawn for managing dyslipidemias: the era of RNA-based therapies. Pharmacol Res 2019;150:104413. 10.1016/j.phrs.2019.104413 [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency. Leqvio (inclisiran). Available from: https://www.ema.europa.eu/en/documents/overview/leqvio-epar-medicine-overview_en.pdf (accessed 1 September 2022)
- 12. US Food and Drug Administration. Leqvio® (inclisiran) injection, for subcutaneous use Initial U.S. Approval: 2021. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=214012 (accessed 1 September 2022)
- 13. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. . Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med 2020;382:1520–1530. 10.1056/NEJMoa1913805 [DOI] [PubMed] [Google Scholar]
- 14. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. . Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. 10.1056/NEJMoa1912387 [DOI] [PubMed] [Google Scholar]
- 15. Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, et al. . Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol 2021;77:1182–1193. 10.1016/j.jacc.2020.12.058 [DOI] [PubMed] [Google Scholar]
- 16. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335–371. 10.1016/s0033-0620(85)80003-7 [DOI] [PubMed] [Google Scholar]
- 17. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. . Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 18. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. . Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J 2020;41:2313–2330. 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 20. Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the cholesterol treatment trialists collaboration. Eur Heart J 2018;39:2540–2545. 10.1093/eurheartj/ehx450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech AC, Kuder JF, et al. . Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 2022;146:1109–1119. 10.1161/CIRCULATIONAHA.122.061620 [DOI] [PubMed] [Google Scholar]
- 22. Steg PG, Szarek M, Bhatt DL, Bittner VA, Bregeault MF, Dalby AJ, et al. . Effect of alirocumab on mortality after acute coronary syndromes. Circulation 2019;140:103–112. 10.1161/CIRCULATIONAHA.118.038840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]
- 24. Gaba P, Bhatt DL, Giugliano RP, Steg PG, Miller M, Brinton EA, et al. . Comparative reductions in investigator-reported and adjudicated ischemic events in REDUCE-IT. J Am Coll Cardiol 2021;78:1525–1537. 10.1016/j.jacc.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 25. Easton JD, Denison H, Evans SR, Knutsson M, Amarenco P, Albers GW, et al. . Estimated treatment effect of ticagrelor versus aspirin by investigator-assessed events compared with judgement by an independent event adjudication committee in the SOCRATES trial. Int J Stroke 2019;14:908–914. 10.1177/1747493019851282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.