FIGURE 1.
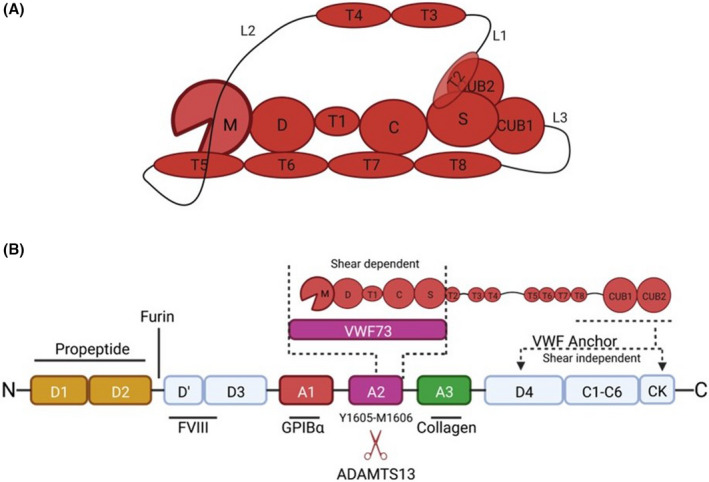
ADAMTS13 binding to VWF and domain organization. A, ADAMTS13 proximal domains include the metalloprotease domains (M), disintegrin‐like domain (D), type‐1 thrombospondin domain (T), cysteine‐rich domain (C), and spacer domain (S). The distal CUB domains are connected to the proximal domains through seven additional type‐1 thrombospondin domains and three linker regions, which provide ADAMTS13 with conformational flexibility. ADAMTS13 adopts a closed conformation in the absence of VWF binding in which the C‐terminal CUB domains bind to the VWF‐binding exosite on the spacer domain. The closed conformation is facilitated by inherent flexibility provided by the linker regions connecting T2‐T3, T4‐T5, and T8‐CUB1. B, ADAMTS13 binds to VWF in both a shear‐independent and a shear‐dependent mechanism. The CUB domains bind to the D4‐CK interval of VWF in a shear‐independent mechanism, which positions MDTCS near the A2 domain. This binding interaction facilitates localization of ADAMTS13 to VWF strings under flow. Upon shear‐activation of the substrate, the spacer, cysteine‐rich, and disintegrin‐like domains bind to the unfolded VWF A2 domain, stabilizing its denatured state and facilitating proteolysis of the Tyr1605‐Met1605 scissile bond by the metalloprotease domain. VWF73, comprising residues Asp1596‐Arg1668 of VWF, is a biochemical tool used to study ADAMTS13 activity in the absence of shear, and only engages the proximal MDTCS domains of ADAMTS13.
