FIGURE 2.
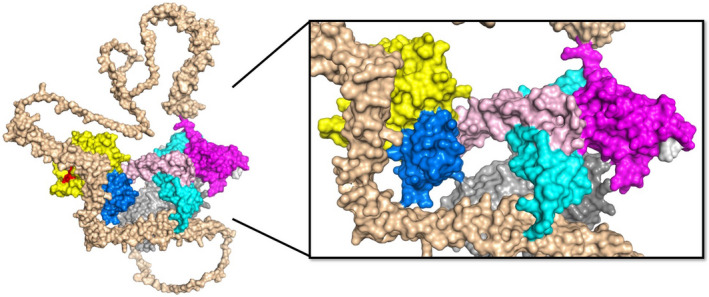
AlphaFOLD2 prediction of full length ADAMTS13 structure. The predicted three‐dimensional structure for full‐length human ADAMTS13 was obtained from AlphaFOLD2 (https://alphafold.ebi.ac.uk/). The domains are indicated as follows: metalloprotease (yellow), disintegrin‐like (blue), TSP1‐1 (rose), cysteine‐rich (cyan), spacer (magenta), TSP1‐2 to TSP1‐8 (wheat), CUB1 (light gray), CUB2 (dark gray). The RRY motif within the spacer domains (white) is primarily associated with autoantibodies in patients with immune TTP and partially occupies the suspected binding site for the CUB domains, but this intramolecular interaction is not represented in this model. Long linker regions extend outward following TSP1‐4 and TSP1‐8, providing flexibility to ADAMTS13 that may facilitate interdomain contacts that promote a closed conformation and confer global latency. This model predicts multiple long‐range interactions between distal TSP1 domains and proximal metalloprotease, disintegrin‐like, and cysteine‐rich domains that may lead to novel hypotheses for the study of ADAMTS13 regulation. (PDB: AF‐Q76LX8‐F1)
