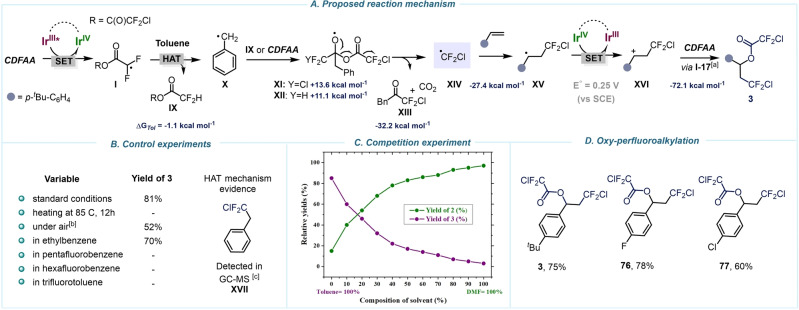
Scheme 6.
Mechanistic studies towards formation of 3. A) Proposed mechanism for oxy‐perfluoroalkylation of olefins. B) Key experiments. C) Competition experiments. D) Oxy‐perfluoroalkylation scope. General conditions: 4‐tert‐butylstyrene (1 equiv), fac‐Ir(ppy)3 (1 mol%), CDFAA (2 equiv), toluene (0.17 M), blue LEDs, rt, 12 h, GC yields. [a] For structure I‐17, see Supporting Information, page S52. [b] Reaction was performed under air. [c] See Supporting Information for details, page S47. Relative Gibbs free‐energies (ΔG) were calculated at 298 K at the CPCM(Solvent)/M06‐2X/GD3/def2‐TZVP//M06‐2X/GD3/def2‐TZVP level of theory.