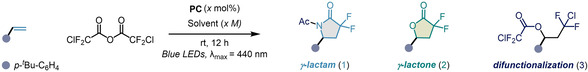
Table 1.
Reaction development and optimization.
|
| ||||||
|---|---|---|---|---|---|---|
|
Entry |
PC (1 mol%) |
Solvent |
Concentration [M] |
1 (%)[a] |
2 (%)[a] |
3 (%)[a] |
|
1 |
[Ir‐1] |
MeCN |
0.10 |
56 |
5 |
– |
|
2 |
[Ir‐1] |
MeCN |
0.03 |
92 (90)[e] |
2 |
– |
|
3 |
[Ru‐1] |
MeCN |
0.03 |
4 |
– |
– |
|
4 |
[Ir‐2] |
MeCN |
0.03 |
18 |
– |
– |
|
5 |
[Ir‐3] |
MeCN |
0.03 |
88 |
– |
– |
|
6 |
[Cu‐1] |
MeCN |
0.03 |
80 |
4 |
– |
|
7 |
[Ir‐1] |
MeCN[b] |
0.10 |
32 |
13 |
– |
|
8 [c] |
[Ir‐1] |
MeCN |
0.03 |
64 |
10 |
– |
|
9 |
[Ir‐1][d] |
MeCN |
0.03 |
83 |
6 |
– |
|
10 |
[Ir‐1] |
DCM |
0.03 |
– |
30 |
53 |
|
11 |
[Ir‐1] |
DMF |
0.03 |
– |
80 |
3 |
|
12 |
[Ir‐1] |
DMF |
0.17 |
– |
86 (81)[e] |
3 |
|
13 |
[Ir‐1] |
DMA |
0.03 |
– |
6 |
– |
|
14 |
[Ir‐1] |
CHCl3 |
0.03 |
– |
56 |
28 |
|
15 |
[Ir‐1] |
Dry Et2O |
0.03 |
– |
9 |
75 |
|
16 |
[Ir‐1] |
Toluene |
0.03 |
– |
16 |
76 |
|
17 |
[Ir‐1] |
Toluene |
0.17 |
– |
14 |
81 (75)[e] |
|
18 |
[Ir‐1] |
THF : CHCl3 (1 : 1) |
0.03 |
– |
44 |
14 |
|
| ||||||
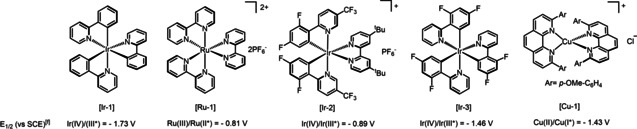
[a] General conditions: 4‐tert‐butylstyrene (0.5 mmol, 1 equiv), catalyst (1.0 mol %), and CDFAA (2 equiv), solvent (x M), 350 W blue LEDs, rt, 12 h. Yields of 1, 2, and 3 were determined by GC‐MS against an internal standard of n‐decane. [b] H2O (1 equiv) was added. [c] Reaction was performed with 2‐bromo‐2,2‐difluoroacetic anhydride. [d] 2.5 mol % of fac‐Ir(ppy)3. [e] Yields in parentheses represent isolated yields. [f] E 1/2 values were taken from refs. [5, 61, 62].