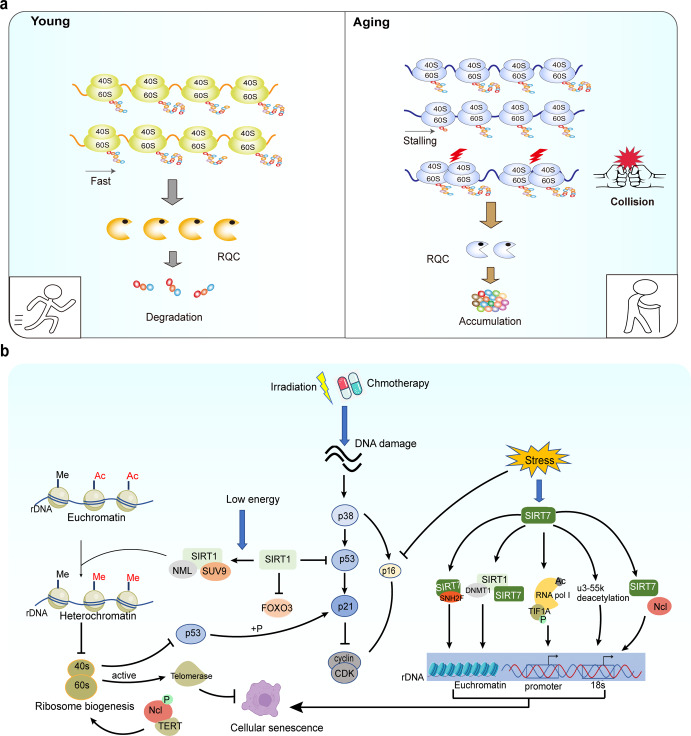
Fig. 7.
a Ribosome-stalling/collision/ribosome dysfunction and aging. The primary clearance pathway for ribosome collisions is the degradation of nascent peptides through ribosomal quality control (RQC). RQC decreases with age, and ribosome-stalling/collision triggers ribosome dysfunction. As a result, ribosome dysfunction leads to increased nascent polypeptides and protein aggregation. b Ribosomes and the signaling pathway of cellular senescence. Cellular DNA damage can be induced by radiation or chemotherapy, which activates p53-dependent stress responses and cause cellular senescence. SIRT1, a member of the longevity protein family, directly affects the activity of key proteins in the senescence pathway through deacetylating transcription factors. Moreover, SIRT1 recruits methylation enzymes to affect ribosome biogenesis by regulating chromatin remodeling, and abnormal ribosome biogenesis can directly affect p53 and telomerase activity to accelerate cellular senescence. Genomic instability of rDNA causes cellular senescence, and SIRT7, a member of the longevity protein family, affects rDNA stability by interacting with chromatin remodeling factors and RNA polymerase. Ncl and SIRT7 interact with proteins, and Ncl binding to telomeric reverse transcriptase affects ribosome biogenesis. Ac acetylation, CDK cyclin-dependent kinases, Me methylation, Ncl nucleolin, p phosphorylation, rDNA ribosomal DNA, RNA pol Ι RNA polymerase Ι