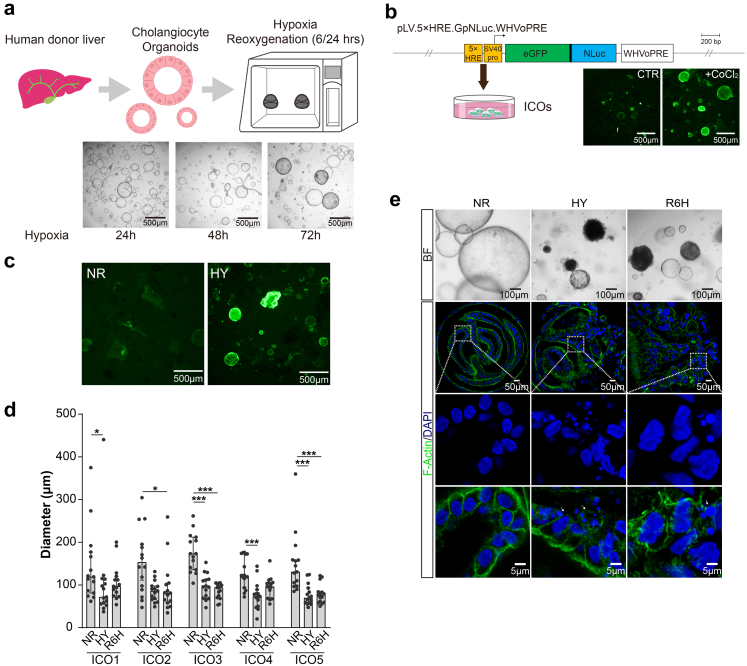
Fig. 1.
Establishment of H/R model using human cholangiocyte organoids. (a) Scheme of the isolation and culture of ICOs from human donor liver specimens and the procedure to establish the H/R model in vitro. (b) Structure of the hypoxia-responsive reporter constructs encoding a constitutively active version of human HIF-1α. ICOs were transduced with lentiviral constructs. Transduced ICOs were incubated with or without CoCl2 (300 μM) for 7 days. Representative fluorescent images of stimulated ICOs were shown (magnification 40×). (c) Transduced ICOs were stimulated with 72-h hypoxia. Representative fluorescent images were shown (magnification 40×). (d) ICOs (n = 5) were stimulated with 72-h hypoxia, followed by 6-h reoxygenation. The diameter of stimulated ICOs was determined in a time-course manner and analyzed using Image J software. (e) ICOs were treated for 72-h hypoxia and 6-h reoxygenation and then fixed and stained with DAPI/Phalloidin. Bright-filed and confocal imaging was shown. Enlarged images from the boxed area are shown in the bottom panel. Dying cells are indicated by arrows. Data are mean ± SD, ∗p < 0.05, ∗∗∗p < 0.001, Kruskal–Wallis test followed by Dunn's post hoc test (d). 5×HRE, 5 copies of the hypoxia-responsive element; eGFP, Aequorea victoria enhanced green fluorescent protein-coding sequence; HY, hypoxia; NLuc, NanoLuc-coding sequence; NR, normoxia; R6h, reoxygenation for 6 h; SV40 pro, minimal simian virus 40 promoters; WHVoPRE, optimized version of the woodchuck hepatitis virus post-transcriptional regulatory element.