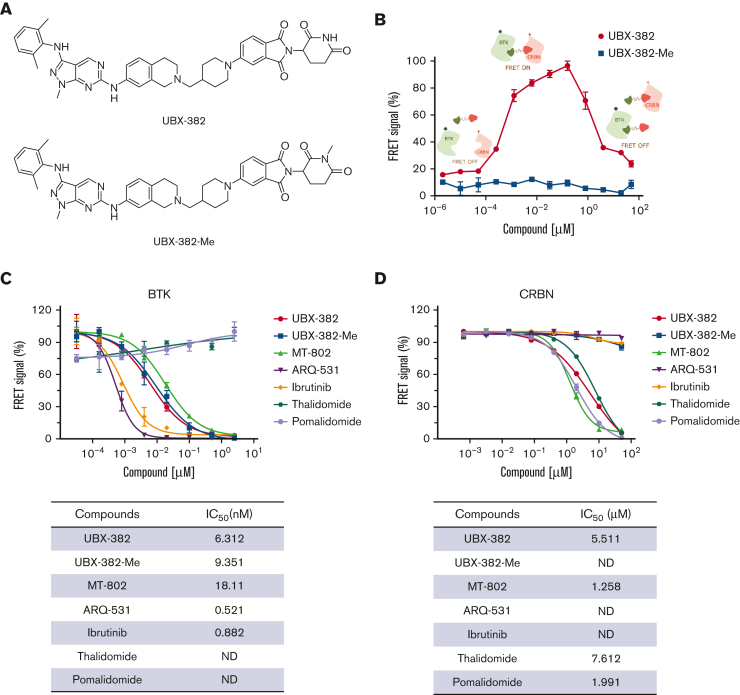
Figure 1.
In vitro target-bindingassay. (A) Chemical structures of UBX-382 and UBX-382-Me. (B) TR-FRET–based in vitro [BTK:PROTAC:CRBN] ternary complex formation assay for UBX-382 and UBX-382-Me. Relative FRET signals represent the normalized mean of FRET signals from 2 replicates. Histidine-tagged (12 nM) and GST-tagged (20 nM) were incubated with 12 different concentrations within the range 0.002048 nM to 50 000 nM for 30 minutes and collected at 620 nm (donor) and 665 nm (acceptor). (C) BTK-target–binding assay for UBX-382 (red circle), UBX-382-Me (blue square), MT-802 (green pyramid), ARQ-531 (purple inverted pyramid), ibrutinib (yellow diamond), thalidomide (hunter green hexagon), and pomalidomide (light purple circle). 50% inhibitory concentration (IC50) values of 6.31, 9.35, 18.11, 0.52, 0.88, not determined (ND), and ND were obtained for UBX-382, UBX-382-Me, MT-802, ARQ-531, ibrutinib, thalidomide, and pomalidomide, respectively. (D) CRBN-target–binding assay for UBX-382 (red circle), UBX-382-Me (blue square), MT-802 (green pyramid), ARQ-531 (purple inverted pyramid), ibrutinib (yellow green diamond), thalidomide (hunter green hexagon), and pomalidomide (light purple circle). IC50 values of 5.51, ND, 1.26, ND, ND, 7.61, and 1.99 were obtained for UBX-382, UBX-382-Me, MT-802, ARQ-531, ibrutinib, thalidomide, and pomalidomide, respectively. Data are represented as the mean values ± standard deviation.