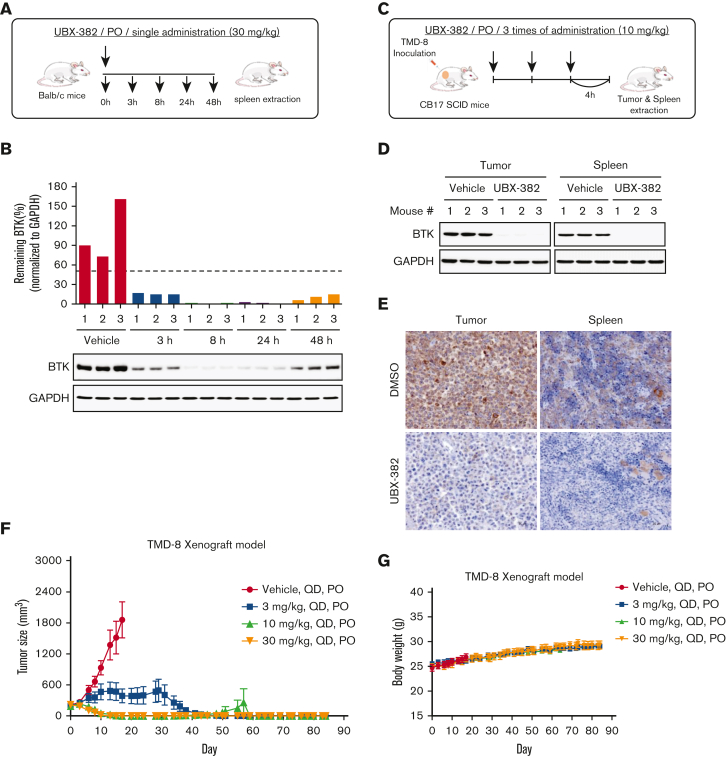
Figure 4.
Evaluation of PD effect of UBX-382 in vivo. (A) Schematic overview of in vivo PD experimental design. (B) BTK levels at each dosing time (3, 8, 24, and 48 hours) were observed in spleen tissues extracted from mice (n = 3 per group). (C) Experimental scheme of in vivo PD experimental design in the xenograft model. (D) Western blot analysis of BTK levels in tumor and spleen samples of vehicle or UBX-382 treated group (n = 3 per group). (E) Immunohistochemical study of BTK levels in TMD-8 tumor and spleen tissues treated with DMSO or UBX-382. Paraffin sections of the tissues were visualized by 3,3-diaminobenzidine staining (n = 3 per group). (F) Graph showing tumor size following UBX-382 administration in the TMD-8 xenograft mouse models, with 9 mice in each group. Xenograft mice were subjected to oral UBX-382 (3, 10, and 30 mg/kg) for 3 weeks. UBX-382 was readministered to the 3 mg/kg group at a concentration of 30 mg/kg for 3 weeks 7 days after the discontinuation of the original dosing. (G) Bodyweight of CB17/severe combined immunodeficient mice treatment groups with TMD-8 xenografts after dosing with vehicle or UBX-382 (n = 9 per group). Data describing tumor growth and body weight are presented as the mean ± SEM. PO, by mouth; QD, daily.