Abstract
The aim of this study was to evaluate the prognostic impact of the F-fluorodeoxyglucose positron emission tomography response at 1 month (M1) and 3 months (M3) after anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in a multicenter cohort of 160 patients with relapsed/refractory large B-cell lymphomas (R/R LBCL). In total, 119 (75%) patients reached M1 evaluation; 64 (53%, 64/119) had a complete response (CR); 91% were Deauville Score (DS) 1-3. Progression-free survival (PFS) and overall survival (OS) were significantly worse in patients with DS-5 at M1, than in patients with DS 1-3 (PFS hazard ratio [HR]=6.37, 95% confidence interval [CI]: 3.5-11.5 vs. OS HR=3.79, 95% CI: 1.7-8.5) and DS-4 (PFS HR=11.99, 95% CI: 5.0-28.9 vs. OS HR=12.49, 95% CI: 2.8-55.8). The 1-year PFS rates were 78.9% (95% CI: 58.9-89.9) for DS-4 at M1, similar to 67.3% (95% CI: 51.8-78.8) for patients with DS 1-3 at M1, very different to 8.6% (95% CI: 1.8-22.4) for DS-5, respectively. Only eight of 30 (26%) patients with DS-4 progressed. Response at M3 evaluated in 90 (57%) patients was prognostic for PFS with lower discrimination (HR=3.28, 95% CI: 1.5-7.0; P=0.003) but did not predict OS (HR=0.61, 95% CI: 0.2-2.3; P=0.45). Patients with a high baseline total metabolic tumor volume (TMTV) >80 mL had worse PFS (HR=2.05, 95% CI: 1.2-3.5; P=0.009) and OS (HR=4.52, 95% CI: 2.5-8.1; P<0.001) than patients with low TMTV. Multivariable analyses identified baseline elevated lactate dehydrogenase, DS-5, CAR T cells at M1 for PFS and baseline elevated lactate dehydrogenase, TMTV >80 mL, and DS-5 at M1 for OS. In conclusion, baseline TMTV and response at M1 strongly predicts outcomes of patients with R/R LBCL undergoing CAR T-cell therapy.
Introduction
CD19-specific chimeric antigenic receptor T cells (CAR T cells) showed impressive efficacy in relapsed and refractory aggressive large B-cell lymphoma (R/R LBCL), including diffuse large B-cell lymphoma not otherwise specified (DLBCL-NOS), transformed follicular lymphoma (tFL), primary mediastinal B-cell lymphoma (PMBCL) or high grade B-cell lymphoma (HBGL) leading to their approval by US Food and Drug Administration (FDA) in 2017 and by European Medicines Agency in 2018 with two products, axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisacel). In the pivotal trials JULIET,1 ZUMA-1,2,3 and TRANSCEND,4 evaluating lisocabtagene maraleucel recently approved by the FDA, as well as in the academic experiences,5–7 complete response (CR) rates range from 40 to 65%. Around 60% of patients will ultimately progress or relapse with progression or relapse events occurring mostly during the first 3 months (M3). Several pretreatment factors have been identified to be associated with relapse or progression, such as elevated lacatate dehydrogenase (LDH), tumor burden measured either by the diameter of the biggest lesion on computerized tomography (CT)-scan or by the total metabolic tumor volume (TMTV) on positron emission tomography (PET)-CT, and more than one extranodal sites involved.6–9
However, little is known about the prognostic value of the response assessed by PET and its time course after CAR T-cell infusion. It has been shown in ZUMA-1 and JULIET trials that reaching complete metabolic response (CMR) or partial metabolic response (PMR) according to Lugano criteria M3 after infusion is predictive of sustained response and had similar outcome.1,3 Although the median time to response is around 1 month (M1) in pivotal trials and real-world experiences,1,2,4,7 the prognostic value of PET assessment after CAR T-cell infusion is under investigation,10 and the conversion rate of response from M1 to M3 post CAR T cells is not fully documented. In this study, we investigated the prognostic value of early metabolic response assessed at M1 and M3 after CAR T-cell infusion in a cohort of patients with R/R LBCL treated with anti-CD19 CAR T cells in four centers, the conversion rate of metabolic responses between M1 and M3, as well as the impact of the baseline TMTV on the PET early metabolic response M1 after CAR T-cell infusion.
Methods
Study population and treatment
The study population included consecutive patients presenting with R/R aggressive B-cell lymphomas treated in four Lymphoma Study Association (LYSA) centers with anti-CD19 CAR T-cell therapy, tisa-cel or axi-cel between June 2018 and November 2020. Real-world data were retrospectively collected from the medical charts of these patients by the treating physicians. Histologic diagnoses were reviewed by expert pathologists (VM). Eligibility for CAR T-cell therapy required relapsed or refractory disease after at least two lines of prior therapy. Bridging therapy was performed at the discretion of physicians. All patients had to perform baseline PET before the procedure of lymphodepletion and CAR T-cell infusion. Association of fludarabine and cyclophosphamide was uniformely used as lymphodepletion preconditioning at the doses recommended for tisa-cel or axi-cel. Patients were discharged from hospital after 10 days if no serious toxicity occurred. Response assessment was performed by clinical and imaging evaluation with CT-scan and PET-CT at M1 +/-5 days (M1 evaluation) and M3 +/- 5 days (M3 evaluation) after CAR T-cell infusion in all patients, except patients with progression before time assessment considered as non-evaluable.
Metabolic evaluation
Analysis of PET-CT images was performed by expert nuclear medicine physicians (LV, PO, CT-V, and CM) to calculate several parameters including the total metabolic tumor volume (TMTV) at baseline, the Deauville score (DS) and the ΔSUVmax at M1 and M3.
The TMTV was computed using the 41% maximum standardized uptake value threshold method for each individual tumor lesion as reported.11
The DS was assessed as follows12 (i.e., 1: no uptake; 2: uptake < mediastinum; 3: uptake > mediastinum but < liver; 4: uptake moderately higher > than liver; 5: uptake markedly higher than liver and/or new lesions). Scores 1, 2 and 3 are considered to represent CMR. Scores 4 and 5 are categorized as PMR, no metabolic response (NMR), stable metabolic disease (SMD), progressive metabolic disease (PMD) accordingly.
CR was assessed if a biopsy performed at the hot site scored DS-4 or DS-5 showed no tumoral infiltration, or in case of a calcified region within the tumoral site. Partial response (PR), stable disease (SD), and progressive disease PD were assessed based on the integration of PET data and radiological data.12
In order to assess the ΔSUVmax between baseline and M1, the hottest tumor in any region or organ was used for comparison, even if its location differed from the initial hottest tumor in baseline PET-CT.13
Statistical analysis
Summary statistics (i.e., median, interquartile range [IQR], and percentages) are reported. PFS was measured from the date of CAR T-cell infusion to the date of death from any cause, disease relapse, or progression. Overall survival (OS) was calculated from the date of CAR T-cell infusion until the date of death from any cause. OS and PFS were estimated using the Kaplan-Meier method. Analyses of ΔSUVmax used landmark at M1, with time-dependent area under the receiver operating characteristic curve computed to determine the best cut-off value to predict progression within the next 12 months. Multivariate analysis was performed using Cox proportional hazard model stratified on the variable ‘center’ and adjusted on the CAR T-cell treatment. All P-values were 2-sided, where P<0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.3. This analysis was approved from the local ethics committee.
Results
Patient characteristics
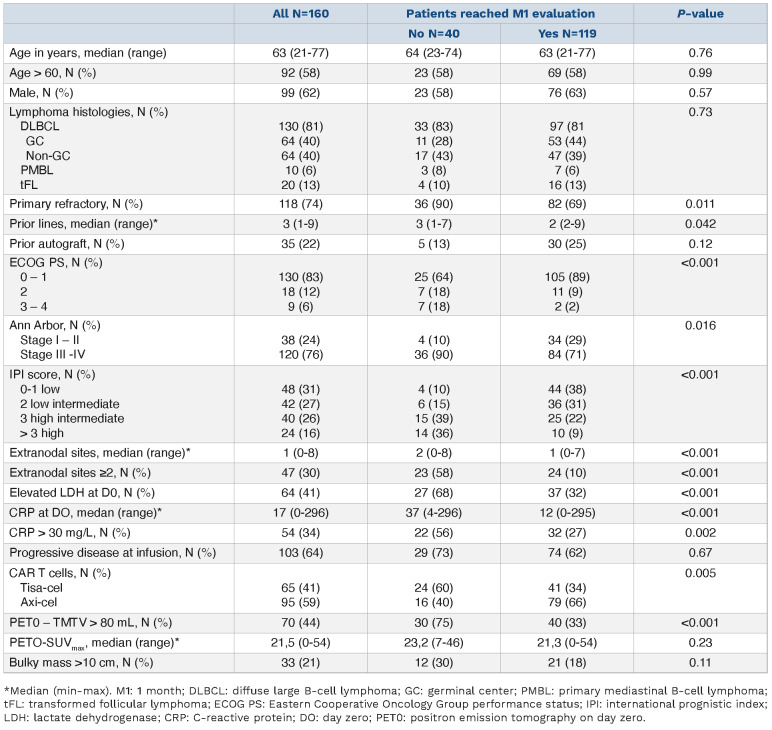
Between June 2018 and November 2020, 160 patients who received either axi-cel (n=95) or tisa-cel (n=65) were enrolled (Figure 1). Clinical and biological characteristics are reported in Table 1.
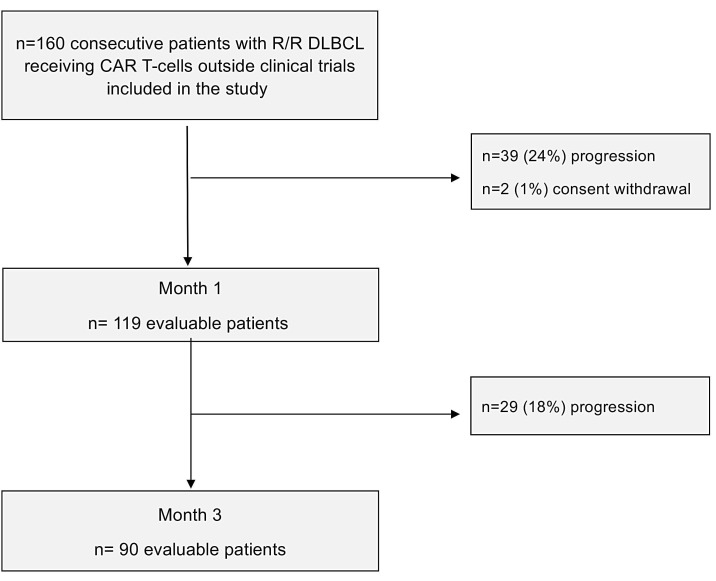
Figure 1.
Flow-chart of the study population.
Response evaluation, and conversions
Thirty-nine patients (24%, 39/160) progressed during the first month and two patients (1%) withdrew their consent. These patients were not evaluable at the first metabolic evaluation (M1). Of the 119 remaining patients (75%) at M1, 64 patients (53%) were classified in CR: 58 patients scored DS 1-3, 5 patients scored DS-4 who had an aspect of calcification on the tumoral site or no tumoral infiltration at the biopsy, one patient scored DS-5 who had similar images at M1 and at the second metabolic evaluation (M3) or a biopsy confirming an histiocytosis infiltration. PR was observed in 39 patients (33%), 22 scored DS-4 and 17 scored DS-5. SD was considered in nine patients (8%): two scored DS-4 and seven scored DS-5. PD was observed in seven patients (6%): one scored DS-4, six scored DS-5. Among the 31 patients scored DS-5 at M1, only one had new lesions.
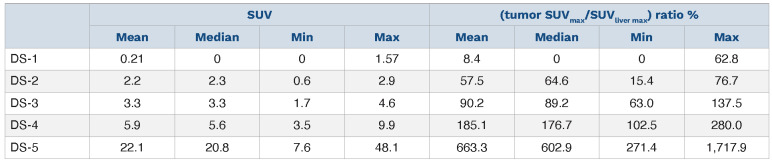
The median (tumor SUVmax/liver SUVmax) ratio x 100 for DS-5 at M1 was 603% (IQR, 434-748%). This was explained by a very high residual tumor uptake. Indeed their median SUVmax was 20.8 (IQR, 15.2-25.4). We can see in the Table 2 that the lower value for DS-4 was 102.5%. This explains that some patients classified DS-4 on the visual basis presented a good outcome. The median (tumor SUVmax/liver SUVmax) ratio for DS-4 patients was 177% (IQR, 148-227%).
Twenty-nine patients (18%, 29/160) progressed between M1 and M3. At M3, 90 patients (57%, 90/160) were evaluable for response. Among them, 64 of 90 (71%) were classified in CR: 58 scored DS 1-3, 5 scored DS-4, 1 scored DS-5. A PR was observed in 15 of 90 (17%): 12 patients scored DS-4 and three scored DS-5. No SD was observed.
A PD was observed in 11 of 90 (12%): all scored DS-5. The median (tumour SUVmax/liver SUVmax) ratio x 100 for DS-4 was 133% (IQR, 124-153%; range, 110-197%). The median (tumor SUVmax/liver SUVmax) ratio x 100 for DS-5 was 400% (IQR, 300-713%; range, 100-1349%).
Analyzing the conversion rate between M1 and M3, among the 39 patients in PR at M1, eight converted to CR including five treated with axi-cel and three with tisa-cel, 15 stayed in PMR and 16 progressed. All patients in SD progressed. Most of patients in CR at M1 (56/64, 88%) did not progress.
Outcome and survivals according to Deauville Score
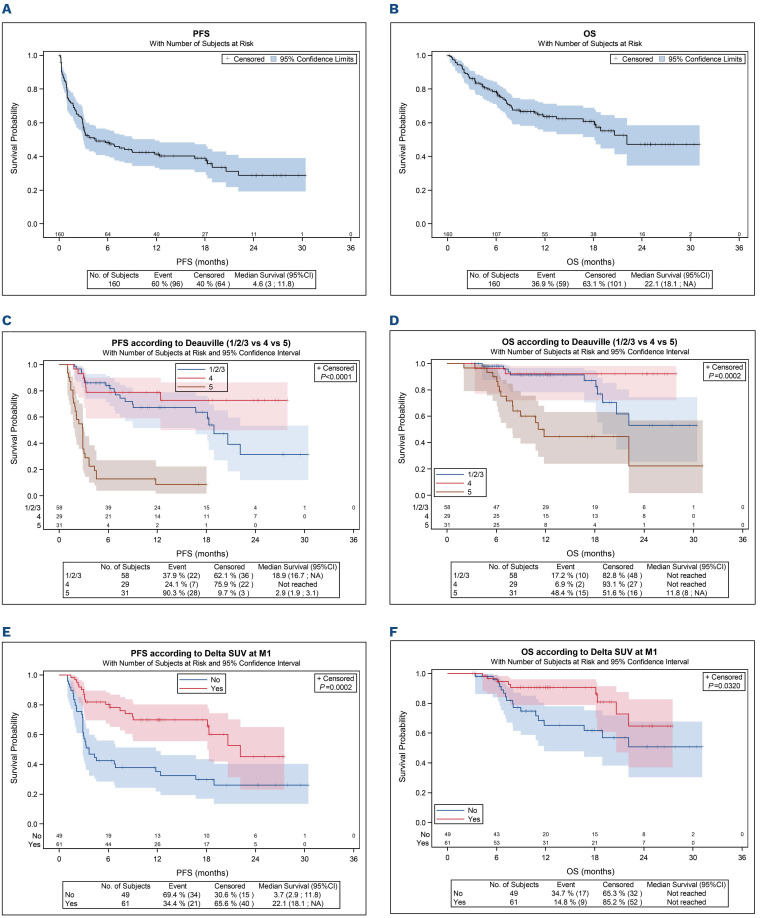
With a median follow-up of 12.6 months, estimated 1 year-OS and PFS were 63.6% (95% CI: 54.7-71.2) and 41.5% (95% CI: 33.3-49.4), with median OS and PFS at 22.1 months and 4.6 months and respectively (Figure 2A and B). For the patients achieving M1 assessment (n=119, 75%), PFS and OS were significantly worse in patients with DS-5 at M1, than in patients with DS 1-3 (PFS HR=6.37, 95% CI: 3.5-11.5 vs. OS HR=3.79, 95% CI: 1.7-8.5) and DS-4 (PFS HR=11.99, 95% CI: 5.0-28.9 vs. OS HR=12.49, 95% CI:2.8-55.8) (Figure 2C and D). The 1-year PFS rates were 78.9% (95% CI: 58.9-89.9) for DS-4 at M1, similar to 67.3% (95% CI: 51.8-78.8) for patients with DS 1-3 at M1, very different to 8.6% (95% CI: 1.8-22.4) for DS-5, respectively. Among the 30 patients scored DS-4, only eight (26%) progressed. For the patients achieving M3 assessment, the patients scored DS-5 had a risk to failure evaluated at 81.2%. The 1-year PFS for patients with DS-5 at M3 was 18.8% compared to 83.5% for patients DS 1/2/3/4 (HR=13.52, 95% CI: 5.9-31.0). No difference was observed for OS between DS 1/2/3/4 and DS-5 (P=0.39). Among the 31 patients with a DS-5 at 1 month, 20 patients progressed before evaluation at M3. Nine more patients were observed with a DS-5 at M3. Most of them (7/8 78%) had a progression/relapse.
Table 1.
Baseline characteristics.
Table 2.
The SUV value and the (tumour SUVmax/SUVliver max) ratio among the Deauville score categories at 1 month.
Prognostic value of ΔSUVmax between positron emission tomography (PET) baseline and PET at 1 month and at 3 months
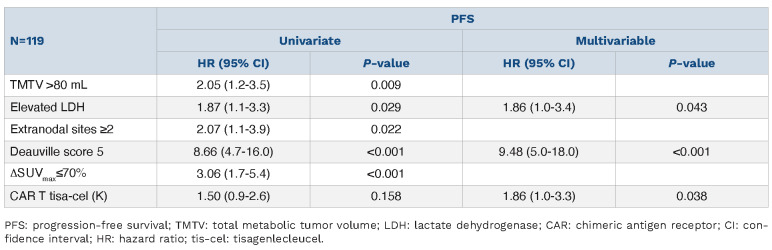
The best cutoff for PET-M1 was 70%.14,15 This cutoff was able to predict failure among patients with evaluable PETCT at M1. Patients with positive PET-M1 had a significant worse PFS than patients with negative PET-M1, with 1-year PFS of 35.1% versus 69.8% (HR=2.73, 95% CI: 1.6-4.7; P<0.001) (Figure 2E), and worse OS (1-year OS at 65.2% vs. 90.6% [HR=2.36, 95% CI: 1.0-5.3; P=0.032]) (Figure 2F). Patients with positive PET-M3 had a significant worse PFS than patients with negative PET-M3, with 1-year PFS of 48.7% versus 84.0% (HR=3.28, 95% CI: 1.5-7.0; P=0.003), but not for OS (HR=0.61, 95% CI: 0.2-2.3; P=0.45).
Analysis of baseline total metabolic tumor volume and response
Seventy of the 160 (44%) patients presented a high baseline TMTV (>80 mL). This parameter was associated with a higher risk of early progression (HR=4.93, 95% CI: 2.9-8.4; P<0.001). Among them, 29 (41%) progressed within 1 month and one patient had less than 1 month of follow-up without event. At M1, 15 were evaluated in CR: 14 scored DS 1-3 and one scored DS-4. Seventeen patients were evaluated in PR: seven scored in DS-4 and ten scored in DS-5. Five patients were evaluated in SD: one scored DS-4 and four scored in DS-5. Three patients were evaluated in PD. Of the 40 remaining patients with high TMTV, 19 (48%) progressed between M1 and M3. At M3, 21 were evaluated, either in CR (n=14): 14 scored DS 1-3, or in PR (n=6): five scored in DS-4 and one scored in DS-5, or in PD (n=1): one scored in DS-5.
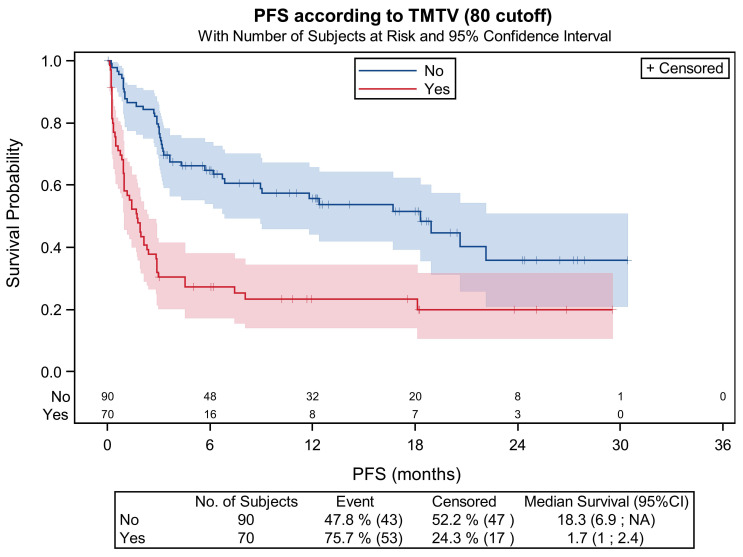
Patients with high TMTV had worse PFS (HR=2.05, 95% CI: 1.2-3.5; P=0.009) and OS (HR=4.52, 95% CI: 2.5-8.1; P<0.001) than patients with low TMTV. The M1 PFS was 58.0% versus 89.9% for patients with low TMTV. The M3 PFS was 30.5% versus 78.7%. The 1-year PFS was 23.4% versus 55.7% (Figure 3). The response at M1 allowed to identify the patients that will relapse even if the baseline TMTV <80 mL was low. Among the 79 patients with low TMTV and metabolic response available at M1, 44 patients (56%) had DS 1-3, 20 patients (25%) had DS-4 and 15 patients (19%) had DS-5. Among the 15 patients with DS-5, 13 (87%) had a relapse/progression or death without progression.
Multivariable analyses and combined models
We performed a multivariable analysis including the parameters known to have a prognostic value at baseline (elevated LDH, high TMTV, ≥2 extranodal sites), and the metabolic response at PET-M1 defined either using the DS (1-4 vs. 5), or ΔSUVmax (<70 vs. ≥70).
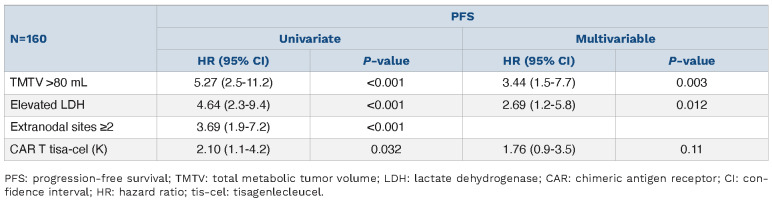
Among the 160 patients, 38 patients (24%) had an early PFS events (<1 month) after CAR T-cell infusion. Patients with high TMTV (HR=2.55; 95% CI: 1.2-5.6; P=0.02), extranodal sites ≥2 (HR=2.10; 95% CI: 1.0-4.3; P=0.045) and elevated LDH (HR=2.80, 95% CI: 1.3-6.0; P=0.008) were significantly associated to a worse early PFS (Table 3). Among the 119 patients free of progression at M1, elevated LDH (HR=1.86, 95% CI: 1.0-3.4; P=0.043), DS-5 at M1 (HR=9.48, 95% CI: 5.0-18.0; P<0.001) and tisa-cel treatment (HR=1.86, 95% CI: 1.0-3.3, P=0.038) were associated to a worse PFS (Table 4).
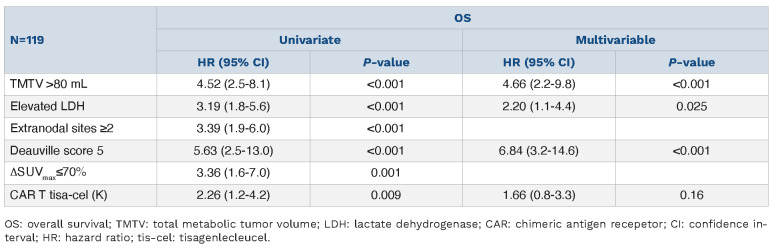
Patients with high TMTV (HR=4.66, 95% CI: 2.2-9.8; P<0.001), elevated LDH (HR=2.20, 95% CI: 1.1-4.4, P=0.025) and DS-5 at M1 (HR=6.84, 95% CI: 3.2-14.6; P<0.001) were associated to a worse OS (Table 5).
Discussion
In this real-world multi-center cohort of 160 patients with R/R LBCL treated with anti-CD19 CAR T cells, we have shown that the metabolic response assessed by FDG PET/CT at 1 month (M1 evaluation), in the 75% of patients reaching this end point, was a strong prognosticator of outcome. PET/CT reported with Deauville criteria with a threshold for positivity set at DS-5 was prognostic of PFS and OS, the risk of PFS being, in the PET-positive patients (DS-5), seven times this observed in PET-negative patients (DS1-4). By contrast, when PET was reported by setting the positivity at DS-4 (bad responders when DS >3) at end of treatment according to Lugano recommendations, M1 evaluation failed to predict outcome. Among the 29 DS-4 patients, only seven had a bad outcome. The residual uptake of the DS-4 patients was high with a median tumor SUVmax/liver SUVmax ratio of 177% ranging from 102.5% to 280%. However that feature is not predictive of a bad outcome in most of these patients treated with CAR T cells. This suggests that the “residual uptake” is not equal to a residual tumoral activity, as it is observed in other lymphomas such as in DLBCL under R-CHOP treatment16,17 or Hodgkin lymphoma under frontline chemotherapy,18,19 and as well as in the context of immunotherapies in solid cancers.20,21 By contrast, the majority of patients who relapsed or died in our study are in the DS-5 group (87% of DS-5 patients had a PFS event and 48% an OS event). These patients had an extremely high residual uptake with a median residual SUVmax six times the liver SUVmax. This suggests that events occur in this specific population for a certain level of residual uptake corresponding to the level suggested as the threshold of DS5, as underlined by another recent study.22 Is this observation related to important aggressiveness and proliferation of the lymphoma or a particular activity of the microenvironment? Further tumoral biological studies are warranted to answer this question. In this setting the ΔSUVmax approach, with a cutoff of 70% defined in populations of DLBCL where positive PET have large range of values of residual uptakes ranging from D-4 just over the liver to D-5 (2 to 3 times over the liver), leads to false-positive results. It does not keep its prognostic value in multivariate analysis.-
Figure 2.
Outcome of the patients. (A) Progression free-survival (PFS) and (B) overall survival (OS) of the 160 patients with relapsed/refractory diffuse large B-cell lymphoma. (C) PFS according to Deauville score (DS) at 1 month (M1) evaluation (DS1-3 vs. DS-4 vs. DS-5). (D) OS according to DS at M1 evaluation (DS1-3 vs. DS-4 vs. DS-5). (E) PFS according to ΔSUVmax (70%) between positron emission tomography on day zero (PET0) and PETM1. (F) OS according to ΔSUVmax (70%) between PET0 and PETM1.
In this series the response evaluated by PET/CT at month 3 by the same approaches (M3 evaluation) in 57% of the group is prognostic of PFS although with lower discrimination between groups but does not predict OS. However, since the majority of these patients (64 patients) were in CMR at M1, M3 evaluation only impacts 17% of the initial group. Therefore, from our results, M1 evaluation seems the preferable slot of time for response assessment in these patients. In addition we have confirmed herein the strong prognostic value of the baseline metabolic tumor volume already reported by us and others7,23,24 as a tool to identify early progressors. Indeed, the high-risk category with TMTV >80 mL at baseline accounted for 70 patients among whom 41% progressed before the M1 evaluation and 11% were in stable or progressive response at M1. At M3, only 21 patients were evaluated, most of them in CMR. However, considering patients with low TMTV <80 mL at baseline (n=79, 49.4% of the cohort), metabolic assessment at M1 based on DS allowed to early identify those that will relapse. 87% of the patients with low TMTV scored DS-5 at M1 will relapse or die. Therefore, metabolic response assessment allowed to predict the outcome of patients whatever the characteristics at baseline, particularly considering low or high TMTV. Thus, TMTV should be considered from our data most as a signal of risk of early progression.
Our data are in accordance with the results of previous series. In ZUMA-1 and JULIET trials, PR patients at M3 after CAR T-cell infusion seem to have an outcome comparable to CR patients, although the number of PR patients at M3 in these studies were very low (n=8 and n=6, respectively).1,3 In TRANSCEND trial, PR as best response is associated with lower PFS than CR.4 As reported in this study, metabolic response assessment at M1 allowed early discriminating patients with poor outcome, with high correlation with M3 findings; this suggests not to delay the risk assessment in these patients, on order to propose alternative or combined treatments.
The response rates as well as the patient outcome reported here are in agreement with those reported in others large clinical trials and academic experiences of CAR T-cell therapy in patients with R/R LBCL. In ZUMA-13, JULIET1, and TRANSCEND4 trials and the recently reported real-world experiences,6,7,9 the overall response rates ranged from 52% to 82%, CR rates from 40% to 65%, and 1-year PFS was estimated around 45%. Similar results are presented here. In all the series, including our present series, progression after CAR T-cells therapy seems to be an early event after infusion in most of the cases, with a median time to progression of 35 days. Indeed 51% of the progressor patients from our study progressed within 1 month. The analysis of our series demonstrates that the best ORR is achieved within the first month after CAR T-cell infusion and with the current follow-up most patients achieving a CMR maintained the CT. Only a few of them (12%) will relapse past M1. In contrary, patients achieving only a PMR at M1 will either ultimately progress, almost half of them (42%), or stay in PMR (38%); only 20% of them will improve to CMR. None of the patients in SMD improved in CMR.
Figure 3.
Progression-free survival considering the total metabolic tumor volume (TMTV) at baseline (high TMTV >80 mL or low TMTV <80 mL).
Table 3.
Early progression-free survival analysis (<1 month).
Table 4.
Progression-free survival analysis (≥1 month).
Table 5.
Overall survival analysis (≥1 month).
Impact of quality of response according to DS on outcome after CAR T-cell therapy in R/R disease has not been described so far. We demonstrated in this study that the evaluation of the metabolic response at M1 is strongly associated with the outcome of the patients.
Baseline factors which correlate with the tumor burden at time of infusion are major predictors of progression after CAR T-cells therapy. Pretreatment serum levels of LDH and proinflammatory markers, including IL-6 and ferritin, PS >1 before treatment are associated with increased risk of progression in several series.6,8 We and other have also shown that high TMTV, defined as a value of more than 80 mL, highly correlates with a high risk of early relapse or progression.7,2 4 Moreover the spread of the disease evaluated by the involvement of more than ≥2 extranodal sites before infusion strongly correlates with early progression.7,25 In this study, we confirm all these parameters as prognosticators, some of them being independently correlated to early failure, PFS, and OS. However, prognosis of high-risk patients at baseline who reached a metabolic response at M1 after infusion, evaluate by DS, appears to have a better outcome than patients that do not reach a CMR at M1 even those with low TMTV. In the near future, these dynamic risk estimates will be further reinforced integrating not only the metabolic response assessment by PET but also the evaluation of the minimal residual disease by circulating tumor DNA (ctDNA) on serial analyses.26,27
In conclusion, baseline TMTV and Deauville-based response at M1 provide important information and are therefore recommended. In contrast, imaging at M3 does not yield prognostic information and could be eliminated unless there is strong clinical suspicion for recurrence.
Acknowledgments
The authors thank the patients and their families, and all the investigators and their staf involved in data collection and analyses. The authors also thank Henry Ortega for his excellent help with Figure 1B.
References
- 1.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell Lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson JS, Palomba LM, Gordon LI, et al . Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 5.Pasquini MC, Hu Z-H, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vercellino L, Di Blasi R Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, Luttwak E, Beyar-Katz O, et al. [18F]FDG PET-CT in patients with DLBCL treated with CAR-T cell therapy: a practical approach of reporting pre- and post-treatment studies. Eur J Nucl Med Mol Imaging. 2021;49(3):953-962. [DOI] [PubMed] [Google Scholar]
- 11.Vercellino L, Cottereau A-S, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48(10):1626-1632. [DOI] [PubMed] [Google Scholar]
- 14.Casasnovas R-O, Ysebaert L, Thieblemont C, et al. FDG-PET-driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study. Blood. 2017;130(11):1315-1326. [DOI] [PubMed] [Google Scholar]
- 15.Le Gouill S, Ghesquières H, Oberic L, et al. Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA. Blood. 2021;137(17):2307-2320. [DOI] [PubMed] [Google Scholar]
- 16.Meignan M, Cottereau A-S. Interim PET in lymphoma: from Deauville to Peking criteria. On the road, again…. Leuk Lymphoma. 2018;59(3):523-525. [DOI] [PubMed] [Google Scholar]
- 17.Toledano MN, Vera P, Tilly H, et al. Comparison of therapeutic evaluation criteria in FDG-PET/CT in patients with diffuse large-cell B-cell lymphoma: prognostic impact of tumor/liver ratio. PLoS One. 2019;14(2):e0211649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casasnovas R-O, Bouabdallah R, Brice P, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomise multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019;20(2):202-215. [DOI] [PubMed] [Google Scholar]
- 20.Dubreuil J, Salles G, Bozzetto J, et al. Usual and unusual pitfalls of 18F-FDG-PET/CT in lymphoma after treatment: a pictorial review. Nucl Med Commun. 2017;38(7):563-576. [DOI] [PubMed] [Google Scholar]
- 21.Gibney GT, Zaemes J, Shand S, et al. PET/CT scan and biopsy-driven approach for safe anti-PD-1 therapy discontinuation in patients with advanced melanoma. J Immunother Cancer. 2021;9(10):e002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhnl A, Roddie C, Kirkwood AA, et al. Early FDG PET response predicts CAR-T failure in large B-cell lymphoma. Blood Adv. 2021;6(1):321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sesques P, Tordo J, Ferrant E, et al. Prognostic impact of 18F-FDG PET/CT in patients with aggressive B-cell lymphoma treated with anti-CD19 chimeric antigen receptor T cells. Clin Nucl Med. 2021;46(8):627-634. [DOI] [PubMed] [Google Scholar]
- 24.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Rocco A, Cuneo A, Di Rocco A, et al. Relapsed/refractory diffuse large B-cell lymphoma patients. A multicenter retrospective analysis of eligibility criteria for car-T cell therapy. Leuk Lymphoma. 2021;62(4):828-836. [DOI] [PubMed] [Google Scholar]
- 26.Sworder B, Kurtz DM, Macaulay C, et al. Circulating DNA for molecular response prediction, characterization of resistance mechanisms and quantification of CAR T-cells during axicabtagene ciloleucel therapy. Blood. 2019;134(Suppl 1):S550. [Google Scholar]
- 27.Frank MJ, Hossain NM, Bukhari A, et al. Monitoring of circulating tumor DNA improves early relapse detection after axicabtagene ciloleucel infusion in large B-cell lymphoma: results of a prospective multi-institutional trial. J Clin Oncol. 2021;39(27):3034-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]