Abstract
Studies have shown that neoadjuvant chemotherapy (NAC) followed by surgical resection improves the survival of patients with esophageal squamous cell carcinoma (ESCC), and that the neutrophil-to-lymphocyte ratio (NLR) nay be a prognostic biomarker in various types of cancer. Despite the noTable changes in the tumor and its microenvironment during NAC, it remains unclear how the NLR changes and which values (before or after NAC) best predict prognosis. The present study aimed to analyze changes in the NLR before and after NAC, and to determine which was a better prognostic factor. This study retrospectively analyzed 338 consecutive patients with ESCC who received NAC followed by curative resection. NLRs before (pre-NLR) and after (post-NLR) NAC were calculated, after which the impact of NAC on NLR, overall survival (OS) and recurrence-free survival (RFS), as well as the relationship between hematological toxicities and NLR, was evaluated. Cutoff values for pre- and post-NLR were 3.7 and 2.5, respectively. Patients with high post-NLR had a worse OS (P=0.0001) and 3-year RFS (P=0.03) than those with low post-NLR. Multivariate analysis identified high post-NLR, pN1 and clinical response as independent prognostic factors. In conclusion, post-NLR was revealed as a better prognostic factor than pre-NLR for patients receiving NAC followed by surgical resection.
Keywords: chemotherapy, esophageal squamous cell carcinoma, neutrophil-to-lymphocyte ratio
Introduction
Esophageal cancer ranks sixth in cancer deaths, with an estimated 572,000 new cases and 509,000 deaths yearly worldwide (1). Several studies have shown that preoperative chemotherapy with cisplatin and 5-fluorouracil (CF) followed by surgical resection improves survival from esophageal squamous cell carcinoma (ESCC) (2–4) and that combination chemotherapy comprising docetaxel, cisplatin, and 5-fluorouracil (DCF) or Adriamycin, cisplatin, and 5-fluorouracil (ACF) provides better outcomes compared to CF (5–8).
Current evidence has revealed that inflammation is a critical component of tumor progression (9). Failure in controlling the immune system could therefore promote tumor progression. Tumor cells and its surrounding microenvironment promote a systemic inflammatory response that alter circulating neutrophil and lymphocyte counts. Accordingly, recent studies have revealed the neutrophil-to-lymphocyte ratio (NLR), one of the easily calculated marker of systemic inflammation (10), can be a potential prognostic biomarker in various solid tumors (11–15), with most studies agreeing with the correlation between an increase in the NLR and worse prognosis.
The triplet regimens, ACF and DCF, have shown promise for ESCC, exhibiting higher response rates but more frequent severe hematological toxicities compared to conventional CF, with DCF and ACF achieving histopathological tumor response rates of 42 and 18%, respectively (5). Furthermore, Grade 3/4 leukopenia and neutropenia were observed in 72.5 and 90% of patients receiving DCF, respectively (16). Although these findings suggest that the tumor and its surrounding microenvironment change dramatically during chemotherapy (17), it remains unclear whether NLR changes before and after chemotherapy. Recently, Hoshino et al reported NLR change, which was calculated NLR after NAC/NLR before NAC, was identified as a significant prognosis predictor (18). In this previous study, 209 patients who underwent NAC followed by thoracic esophagectomy for esophageal cancer were enrolled, DCF was performed 35 (16.7%) patients. The patients with NLR change below 0.55 had a significantly better Overall survival (OS) and recurrence-free survival (RFS) than those with NLR change above 0.55. However, no consensus has been reached regarding which NLR value (i.e., before or after chemotherapy) could better predict prognosis.
Up to our knowledge, the present study firstly aimed to analyze changes in NLR during triplet chemotherapy and surgical intervention in 338 consecutive ESCC patients and to determine whether the NLR upon starting neoadjuvant chemotherapy (NAC) (pre-NLR) or that upon subsequent esophagectomy (post-NLR) would be a better prognostic factor.
Materials and methods
Patients
This retrospective analysis included data from 441 consecutive patients who were histologically diagnosed with primary thoracic ESCC and underwent esophagectomy at Osaka University Hospital between 2010 and 2016. The present report analyzed 338 patients retrospectively after excluding patients who satisfied the following criteria: i) Had previous or other concomitant cancers, ii) did not receive NAC, ii) received neoadjuvant radiotherapy, iv) received noncurative resection, and v) incomplete or inexhaustive follow-up data. All clinicopathological data were retrieved from medical records at Osaka University Hospital. The clinicopathologic findings were classified according to the UICC-TNM Classification, seventh edition (19).
Calculation of pre- and post-NLR
NLR was calculated as the ratio of the number of neutrophils to the number of lymphocytes collected in a blood test. Each patient underwent NLR counting twice (i.e., before and after NAC). Pre- and post-NLR were calculated in 1–7 days before starting NAC and subsequently after esophagectomy, respectively.
Outcomes
OS and RFS were evaluated. OS was calculated from the date of surgery until an event or last known date of follow-up, whereas RFS was calculated from the date of surgery until a recurrence event, death, or last known date of follow-up, whichever occurred first. Moreover, clinical and histopathological responses were assessed. Clinical response was evaluated through computed tomography (CT) and esophagoscopy based on the criteria of Japan Esophageal Society (19). Briefly, complete response (CR) was defined as the complete disappearance of all evidence of the tumor, including negative biopsy results; partial response (PR) was defined as a ≥50% decrease; sTable disease was defined as a <50% reduction and <25% increase; and progressive disease was defined as a ≥25% increase. Responses were determined after NAC. Histopathological tumor response was evaluated according to the histological criteria of the Japanese Esophageal Society (20) and was classified into five categories according to the proportion of tumor degeneration and necrosis: Grade 3 (markedly effective; no viable cancer cells); grade 2 (moderately effective; viable cancer cells accounting for less than 1/3 of tumor tissue, while other cancer cells showed severe degeneration or necrosis); grade 1 (slightly effective; apparently viable cancer cells accounting for 1/3 or more of tumor tissue, but some evidence of degenerating cancer tissue or cells was present), and grade 0 (ineffective; denoting no discernible therapeutic effect on cancer tissue or cells). Grade 1 lesions can also be subclassified into grade 1a (viable cancer cells accounting for 2/3 or more of tumor tissue) and grade 1b (viable cancer cells accounting for 1/3 or more, but less than 2/3, of tumor tissue). Furthermore, hematological toxicities occurring after triplet chemotherapy were determined according to the Common Terminology Criteria for Adverse Events (CTCAE) (21).
Treatment
All patients included in the present study were treated with two or three cycles of NAC, ACF, or DCF. ACF chemotherapy consisted of Adriamycin 35 mg/m2 and cisplatin 70 mg/m2 on day 1 and 5-FU 700 mg/m2/day on days 1–7 every 4 weeks (22). DCF chemotherapy consisted of docetaxel 70 mg/m2 and cisplatin 70 mg/m2 on day 1 and 5-FU 700 mg/m2/day on days 1–5 every 3 weeks (5–8). Our standard procedure was subtotal esophagectomy performed via a right thoracotomy or video-assisted thoracoscopic surgery with two- or three-field lymphadenectomy (23,24).
Patient follow-up
Patients who undergo esophagectomy at our institution were followed up through tumor markers and CT scan every 3 months during the first 2 years and every 4–6 months for the next 3 years, and annually after 5 years (23,25). Upper gastrointestinal endoscopy was also performed once a year, with PET-CT being performed as necessary. The last follow-up was in December 2019, which included verification of the clinical records.
Statistical analysis
Categorical and continuous data were compared using the χ2 test and the Mann-Whitney U test, respectively. Cutoff values were determined using the receiver operating characteristic (ROC) curve. A matched pairs t-test was used to determine significant differences between pre- and post-NLR. The Kaplan-Meier method was used to estimate OS and RFS after surgery, while the generalized Wilcoxon test was used to compare the groups and assess prognostic values. Multivariate analyses using Cox regression modeling and logistic regression with variables having P-values less than 0.1 were performed. The statistical significance of each model was set at P<0.05. All statistical analyses were performed using JMP Pro ver 14.0.0.
Results
Numerical data for pre- and post-NLR
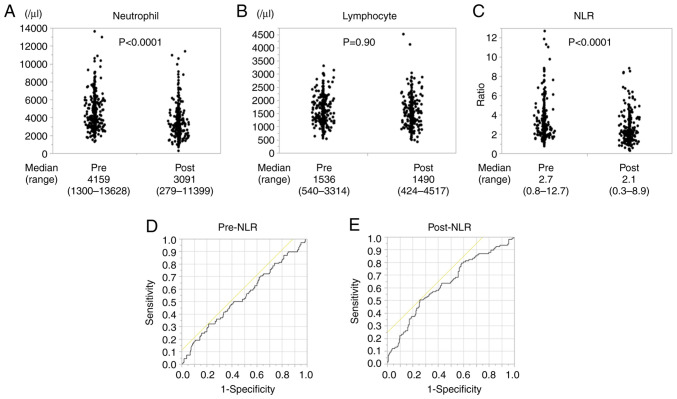
While neutrophil count significantly decreased after NAC (Fig. 1A), no change in lymphocyte count was noted (Fig. 1B). Post-NLR was significantly lower than pre-NLR 2.1 (0.3-19.2) vs. 2.7 (0.8-12.7); P<0.0001; Fig. 1C). A comparison of matched pairs of pre- and post-NLR also showed that NLR decreased significantly after chemotherapy (P<0.0001).
Figure 1.
(A) Numerical data for pre- and post-neutrophil count (n=338). (B) Numerical data of pre- and post-lymphocyte count (n=338). (C) Numerical data for pre- and post-NLR (n=338). (D) ROC curve analysis for the optimal cutoff pre-NLR value which discriminated between survival and death 3 years after operation. (E) ROC curve analysis for the optimal cutoff post-NLR value which discriminated between survival and death 3 years after operation. NLR, neutrophil-to-lymphocyte ratio; ROC, receiver operating characteristic.
Optimal cutoff values for pre- and post-NLR
To evaluate which NLR was a better biomarker, cutoff values for pre- and post-NLR were set based on the ROC. Accordingly, pre-NLR had a cutoff value of 3.7, which discriminated between survival and death 3 years after operation, with an area under the curve of 0.54662 and a sensitivity and specificity of 78.7 and 32.4%, respectively (Fig. 1D). Post-NLR had a cutoff value of 2.5, with an area under the curve of 0.63255 and a sensitivity and specificity of 51.0 and 74.3%, respectively (Fig. 1E). We also analyzed the ROC which discriminated between clinical response PR-CR and PD-SD. Accordingly, pre-NLR had a cutoff value of 5.5 with an area under the curve of 0.48864 and a sensitivity and specificity of 94.1 and 12.5%, respectively. Post-NLR had a cutoff value of 2.5, with an area under the curve of 0.56634 and a sensitivity and specificity of 47.1 and 70.4%, respectively (Fig. S1A and B). Considering these separate analyses, we set the optimal cut off value at 3.7 for pre-NLR and 2.5 for post-NLR which were determined by the ROC curve of survival and death 3 years after operation for two reasons. Firstly, the ROC curve of survival and death 3 years after operation had better sensitivity and specificity than that of clinical response PR-CR and PD-SD. Secondly, the NLR cutoff values generally ranged from 2 to 5 (26).
Characteristics of patients
A total of 338 patients were included in this study. Based on the cutoff value, 87 (26%) and 251 patients (74%) were assigned to low and high pre-NLR group, while 111 (33%) and 227 (67%) were assigned to low and high post-NLR group, respectively. Table I compares the clinical characteristics between both pre- and post-NLR groups. Accordingly, the high groups had lower BMI, higher neutrophil count, and lower lymphocyte count compared to the low groups. Both pre- and post-NLR were not significantly correlated with age, gender, cStage and American Society of Anesthesiologists physical status. Among the 338 patients, 259 (77%) received DCF, while 79 (23%) received ACF as NAC, with almost the same rates observed in pre- and post-NLR.
Table I.
Patient and tumor characteristics, based on pre- and post-NLR.
| Pre-NLR | Post-NLR | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | High (n=87) | Low (n=251) | P-value | High (n=111) | Low (n=227) | P-value |
| Age, years | 0.18 | 0.07 | ||||
| Median (range) | 69 (35–82) | 67 (38–83) | 67 (35–83) | 69 (38–82) | ||
| Sex | 0.06 | 0.10 | ||||
| Male | 82 | 217 | 103 | 195 | ||
| Female | 5 | 34 | 8 | 31 | ||
| Ethnicity | ||||||
| Asian | 87 | 251 | 111 | 227 | ||
| Others | 0 | 0 | 0 | 0 | ||
| BMI, kg/m2 | 0.04 | 0.04 | ||||
| Median (range) | 20.0 (15.0-28.8) | 21.3 (14.8-29.7) | 20.5 (14.9-29.7) | 21.3 (14.8-28.7) | ||
| Location | 0.38 | 0.77 | ||||
| Ut | 13 | 49 | 18 | 44 | ||
| Mt | 46 | 112 | 53 | 105 | ||
| Lt | 28 | 90 | 40 | 78 | ||
| Histology | 0.86 | 0.91 | ||||
| Poor | 6 | 16 | 7 | 15 | ||
| Well/moderate | 81 | 235 | 104 | 212 | ||
| cT a | 0.31 | 0.78 | ||||
| 1 | 1 | 18 | 6 | 13 | ||
| 2 | 10 | 57 | 15 | 52 | ||
| 3 | 56 | 147 | 75 | 128 | ||
| 4 | 20 | 29 | 15 | 34 | ||
| cN a | 0.18 | 0.79 | ||||
| 0 | 24 | 60 | 23 | 61 | ||
| 1 | 42 | 140 | 65 | 117 | ||
| 2 | 21 | 45 | 21 | 45 | ||
| 3 | 0 | 6 | 2 | 4 | ||
| cM a | 0.76 | 0.75 | ||||
| 0 | 11 | 35 | 95 | 197 | ||
| 1 | 76 | 216 | 16 | 30 | ||
| cStage a | 0.10 | 0.53 | ||||
| I | 4 | 34 | 9 | 29 | ||
| II | 18 | 52 | 21 | 49 | ||
| III | 54 | 129 | 65 | 118 | ||
| IV | 11 | 36 | 16 | 31 | ||
| WBCs, µl | <0.0001 | <0.0001 | ||||
| Median (range) | 8,130 | 5,930 | 6,130 | 4,880 | ||
| (4,210–16,840) | (2,590–13,810) | (2,720–13,750) | (1,140–10,800) | |||
| Neutrophils, µl | <0.0001 | <0.0001 | ||||
| Median (range) | 6,132 | 3,704 | 4,141 | 2,631 | ||
| (3,198–13,628) | (1,300–9,087) | (1,778–11,399) | (279–6,405) | |||
| Lymphocytes, µl | <0.0001 | <0.0001 | ||||
| Median (range) | 1,169 | 1,698 | 1,212 | 1,601 | ||
| (540–2,360) | (729–3,314) | (424–2,142) | (670–4,517) | |||
| ASA PS | 0.37 | 0.25 | ||||
| 1 | 11 | 40 | 18 | 33 | ||
| 2 | 69 | 200 | 84 | 185 | ||
| 3 | 7 | 11 | 9 | 9 | ||
| Preoperative chemotherapy | 0.77 | 0.78 | ||||
| DCF | 68 | 191 | 84 | 175 | ||
| ACF | 19 | 60 | 27 | 52 | ||
NLR, neutrophil-to-lymphocyte ratio; BMI, body mass index; Ut, upper thoracic esophagus; Mt, middle thoracic esophagus; Lt, lower thoracic esophagus; ASA PS, American Society of Anesthesiologists physical status; DCF, docetaxel, cisplatin, and 5-fluorouracil; ACF, adriamycin, cisplatin, and 5-fluorouracil.
UICC 7th.
Hematological toxicities
To determine how chemotherapy directly influenced change in peripheral leukocyte count and evaluate its correlation with NLR, hematological toxicities were compared between low and high pre- and post-NLR groups (Table II). Interestingly, the high group had a significantly higher incidence of grade 3/4 lymphopenia than the low group in both pre-NLR (44% vs. 27%; P=0.0073) and post-NLR (49% vs. 23%; P<0.0001), whereas no difference in the incidence of neutropenia was noted (pre-NLR: 91% vs. 92%; P=0.65 and post-NLR: 91% vs. 92%; P=1.0). Furthermore, the high pre-NLR group had a significantly higher incidence of anemia than the low group (16% vs. 8%; P=0.04). Aa similar tendency, albeit not significant, was observed in the post-NLR group (14% vs. 8%; P=0.08). No differences in frequencies of other hematological toxicities, such as leukopenia (pre-NLR: 86% vs. 82%; P=0.41, post-NLR: 87% vs. 81%; P=0.17), febrile neutropenia (pre-NLR: 47% vs. 48%; P=0.12, post-NLR: 50% vs. 47%; P=0.73), and thrombocytopenia (pre-NLR: 2% vs. 7%; P=0.90, post-NLR: 9% vs. 4%; P=0.14) were observed.
Table II.
Hematological toxicities NCI-CTC grade 3/4.
| Pre-NLR | Post-NLR | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Parameter | High (n=87), n (%) | Low (n=251), n (%) | P-value | High (n=111), n (%) | Low (n=227), n (%) | P-value |
| Leukopenia | 75 (86) | 206 (82) | 0.41 | 97 (87) | 184 (81) | 0.17 |
| Neutropenia | 79 (91) | 232 (92) | 0.65 | 102 (92) | 209 (92) | 1.0 |
| Lymphopenia | 38 (44) | 69 (27) | 0.0073 | 54 (49) | 53 (23) | <.0001 |
| Anemia | 14 (16) | 20 (8) | 0.04 | 16 (14) | 18 (8) | 0.08 |
| Thrombocytopenia | 2 (2) | 18 (7) | 0.12 | 10 (9) | 10 (4) | 0.14 |
| Febrile neutropenia | 41 (47) | 121 (48) | 0.90 | 55 (50) | 107 (47) | 0.73 |
NLR, neutrophil-to-lymphocyte ratio; NCI-CTC, National Cancer Institute Common Toxicity Criteria.
Clinical and histopathological response
With regard to clinical response to NAC, 67 (77%) and 203 (81%) achieved PR or CR in the high and low pre-NLR group, respectively (Table III). No significant relationship was observed between clinical response and pre-NLR (P=0.44). On the other hand, 190 (83%) and 80 (72%) patients showed PR or CR in the low and high post-NLR, respectively. Patients with high post-NLR had significantly worse clinical response (P=0.01). Similarly, no significant relationship was observed between histopathological response rate and pre- or post-NLR.
Table III.
Clinical response and histopathological response rate.
| Pre-NLR | Post-NLR | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Parameter | High (n=87) | Low (n=251) | P-value | High (n=111) | Low (n=227) | P-value |
| Clinical response | 0.44 | 0.01 | ||||
| PD/SD | 20 | 48 | 31 | 37 | ||
| PR/CR | 67 | 203 | 80 | 190 | ||
| Pathological response | 0.31 | 0.15 | ||||
| Grade 0/1b | 58 | 151 | 75 | 134 | ||
| Grade 2 | 29 | 100 | 36 | 93 | ||
| pTa | 0.38 | 0.28 | ||||
| 0 | 7 | 31 | 10 | 28 | ||
| 1 | 16 | 70 | 22 | 64 | ||
| 2 | 18 | 47 | 20 | 45 | ||
| 3 | 45 | 102 | 58 | 89 | ||
| 4 | 1 | 1 | 1 | 1 | ||
| pNa | 0.91 | 0.31 | ||||
| 0 | 32 | 103 | 41 | 94 | ||
| 1 | 28 | 77 | 42 | 63 | ||
| 2 | 18 | 46 | 18 | 46 | ||
| 3 | 9 | 25 | 10 | 24 | ||
| pM a | 0.12 | 0.22 | ||||
| 0 | 82 | 222 | 103 | 201 | ||
| 1 | 5 | 29 | 8 | 26 | ||
| pStagea | 0.11 | 0.40 | ||||
| 0 | 5 | 27 | 9 | 23 | ||
| I | 16 | 55 | 20 | 51 | ||
| II | 20 | 54 | 26 | 48 | ||
| III | 41 | 86 | 48 | 79 | ||
| IV | 5 | 29 | 8 | 26 | ||
NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
UICC 7th.
Overall and recurrence-free survival
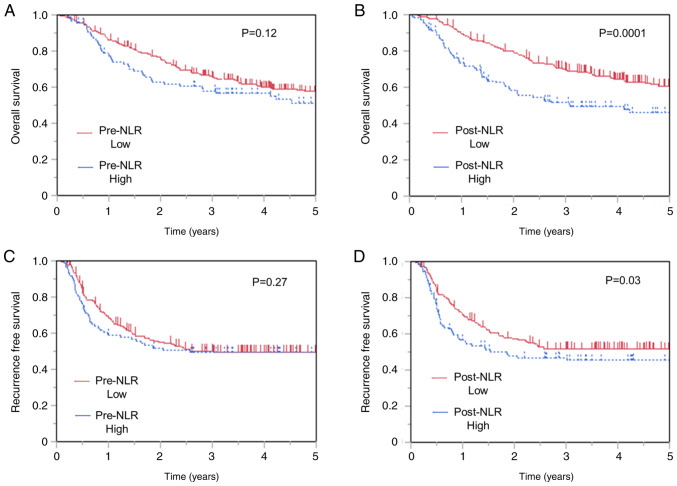
The 3-year OS rates were 58.0 and 66.1% in patients with high and low pre-NLR, respectively. No difference in OS was observed between patients with low and high pre-NLR (P=0.12; Fig. 2A). On the other hand, patients with high post-NLR showed worse OS (Fig. 2B) than those with low post-NLR (P=0.0001), with the 3-year OS of 51.4 and 70.1% in patients with high and low post-NLR patients, respectively. Moreover, no difference in RFS was observed between patients with low and high pre-NLR (P=0.27) (Fig. 2C), whereas those with high post-NLR showed worse RFS than those with low post-NLR (Fig. 2D). The 3-year RFS rates were 46.9 and 51.4% in patients with high and low post-NLR, respectively (P=0.03). Similarly, no difference in RFS was observed between patients with low and high pre-NLR (P=0.27).
Figure 2.
Kaplan-Meier analysis of overall survival classified according to (A) pre-NLR and (B) post-NLR. Kaplan-Meier analysis of recurrence-free survival classified according to (C) pre-NLR and (D) post-NLR (n=338). NLR, neutrophil-to-lymphocyte ratio.
Univariate analysis identified pT (3-4/0-2, P<0.0001), pN (1–3/0, P<0.0001), pM stage (1/0, P=0.02), clinical response (PD-SD/PR-CR, P<0.0001), pathological response (Grade 0-Ib/II, P<0.0001), and post-NLR (≥2.5/<2.5, P=0.0007) as significant factors for OS (Table IV). To determine independent prognostic factors for OS, a multivariate Cox proportional hazards model was performed with variables having P-values of less than 0.1 during univariate analysis. Accordingly, multivariate analysis identified high post-NLR (HR 1.62, 95% CI 1.14-2.28; P=0.008) as independent prognostic factor, along with advanced pN stage (HR 2.34, 95% CI 1.53-3.65; P<0.0001) and worse clinical response (HR 1.70, 95% CI 1.12-2.51; P=0.01).
Table IV.
Univariate and multivariate analysis of overall survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex, male/female | 1.02 (0.62-1.83) | 0.92 | ||
| Age, ≥75/<75 | 1.26 (0.81-1.88) | 0.29 | ||
| BMI, <18.5/≥18.5 | 1.58 (0.99-2.42) | 0.06 | 1.03 (0.63-1.62) | 0.90 |
| Location, Ut/Mt-Lt | 1.16 (0.75-1.73) | 0.50 | ||
| Histology, poor/well or moderate | 1.00 (0.49-1.81) | 1.00 | ||
| Preoperative chemotherapy, DCF/ACF | 0.77 (0.54-1.12) | 0.17 | 0.89 (0.61-1.32) | 0.56 |
| pT, 3-4/0-2a | 2.47 (1.78-3.47) | <0.0001 | 1.29 (0.84-1.98) | 0.25 |
| pN, 1-3/0a | 3.17 (2.17-4.76) | <0.0001 | 2.34 (1.53-3.65) | <.0001 |
| pM, 1/0a | 1.68 (1.08-2.53) | 0.02 | 1.49 (0.88-2.40) | 0.13 |
| Pre-NLR, ≥3.7/<3.7 | 1.25 (0.86-1.78) | 0.23 | ||
| Post-NLR, ≥2.5/<2.5 | 1.81 (1.29-2.52) | 0.0007 | 1.62 (1.14-2.28) | 0.008 |
| Clinical response, PD-SD/PR-CR | 2.32 (1.61-3.27) | <0.0001 | 1.70 (1.12-2.51) | 0.01 |
| Pathological response, Grade 0-1b/2 | 2.71 (1.85-4.08) | <0.0001 | 1.36 (0.83-2.24) | 0.22 |
BMI, body mass index; Ut, upper thoracal esophagus; Mt, middile thoracal esophagus; Lt, lower thoracal esophagus; DCF, docetaxel, cisplatin, and 5-fluorouracil; ACF, adriamycin, cisplatin, and 5-fluorouracil; NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
UICC 7th.
Independent factors for high post-NLR
Univariate analysis identified age (≥75/<75, P=0.0015), BMI (<18.5/≥18.5, P=0.0006), pre-neutrocyte counts (≥6,000/µl/<6,000/µl, P=0.0011), pre-lymphocyte counts (≥1,500/µl/<1,500/µl), P<0.0001), and clinical response (PD-SD/PR-CR, P=0.014) as significant factors for higher post-NLR (Table V). Multivariate logistic regression was then performed using variables having P-values than 0.1 during univariate analysis. Interestingly, higher age (OR 2.36, 95% CI 1.22-4.55; P=0.01), lower BMI (OR 2.51, 95% CI 1.23-5.11; P=0.01), higher pre-neutrocyte counts (OR 3.57, 95% CI 1.87-6.84; P=0.0001), lower pre-lymphocyte counts (OR 0.38, 95% CI 0.22-0.66; P=0.0006), and lymphopenia (OR 2.90, 95% CI 1.64-5.10; P=0.0002) were identified as independent factors for high post-NLR.
Table V.
Univariate and multivariate analysis of post-NLR.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex, male/female | 2.0 (0.90-4.59) | 0.10 | ||
| Age, ≥75/<75 | 2.54 (1.45-4.48) | 0.0015 | 2.36 (1.22-4.55) | 0.01 |
| BMI, <18.5/≥18.5 | 3.16 (1.68-5.98) | 0.0006 | 2.51 (1.23-5.11) | 0.01 |
| Preoperative chemotherapy, DCF/ACF | 0.92 (0.54-1.57) | 0.79 | 0.81 (0.43-1.53) | 0.51 |
| pT, 3-4/0-2a | 1.73 (1.09-2.73) | 0.02 | 1.25 (0.68-2.32) | 0.28 |
| pN, 1-3/0a | 1.2 (0.76-1.93) | 0.48 | 1.05 (0.59-1.89) | 0.71 |
| pM, 1/0a | 0.60 (0.26-1.37) | 0.25 | ||
| Pre-neutrocyte, ≥6,000/µl/<6,000/µl | 2.58 (1.48-4.48) | 0.0011 | 3.57 (1.87-6.84) | 0.0001 |
| Pre-lymphocytes, ≥1,500/µl/<1,500/µl | 0.30 (0.19-0.49) | <0.0001 | 0.38 (0.22-0.66) | 0.0006 |
| Neutropenia, Grade 3-4/1-2 | 0.98 (0.42-2.25) | 1.0 | ||
| Lymphopenia, Grade 3-4/1-2 | 3.10 (1.92-5.04) | <0.0001 | 2.90 (1.64-5.10) | 0.0002 |
| Anemia, Grade3-4/1-2 | 1.96 (0.96-4.00) | 0.07 | 0.90 (0.38-2.16) | 0.82 |
| Clinical response, PD-SD/PR-CR | 0.50 (0.29-0.87) | 0.014 | 0.55 (0.27-1.07) | 0.07 |
| Pathological response, Grade 0-1b/2 | 0.69 (0.43-1.11) | 0.15 | ||
BMI, body mass index; DCF, docetaxel, cisplatin, and 5-fluorouracil; ACF, adriamycin, cisplatin, and 5-fluorouracil; NLR, neutrophil-to-lymphocyte ratio; PR, partial response; CR, complete response; PD, progressive disease; SD, stable disease.
UICC 7th.
Discussion
Up to our knowledge, this has been the first study to compare the prognostic utility of pre- and post-chemotherapy NLR values in 338 consecutive patients receiving triplet neoadjuvant regimens. Accordingly, the present study showed that high post-NLR but not pre-NLR was an independent prognostic factor for OS among those receiving triplet chemotherapy followed by surgical resection. Moreover, high post-NLR was significantly correlated with poor clinical response to NAC.
A meta-analysis including a total of 20 studies consisting of 6457 patients showed a significant association between higher NLR and worse survival in esophageal cancer, subsequently considering NLR as an independent predictive marker (27). However, majority of these previous reports have focused on patients who underwent surgery alone, with only a handful having investigated variations in NLR following NAC. Among such studies, Miyazaki et al identified high NLR before surgery as a significant risk factor for poor prognosis in patients who did or did not undergo NAC for esophageal cancer (28). Despite the availability of similar reports (29–31), it remains unclear whether NAC influences NLR given that the aforementioned studies analyzed patients who both did and did not receive chemotherapy with no unified timing of NLR measurements. Lee et al reported that post-treatment NLR but not pre-treatment NLR was a significant prognostic factor following chemotherapy including gefinitib, gemcitabine, and cisplatin for advanced or metastatic lung adenocarcinoma and that high post-treatment NLR was associated with worse tumor response, higher risk of progression, and greater risk of death (32). The aforementioned results corroborate the findings presented herein, which showed that post-NLR was a potential prognostic marker. The present report suggests that NLR may predict prognosis more precisely by considering the timing of measurements. Interestingly, Hoshino et al focused on NLR change itself, which was calculated NLR after NAC/NLR before NAC (18). NLR change identified as a significant prognosis predictor, the patients with NLR change below 0.55 had a significantly better OS and RFS than those with NLR change above 0.55. Notably, these tendencies increased in patients who underwent DCF in comparison with those who underwent CF. However, their study included a small number of cases, 35 patients who underwent DCF with esophageal cancer. The present report has been the first to directly reveal the impact chemotherapy may have on NLR in more than 300 patients who underwent triplet chemotherapy for ESCC.
The mechanisms by which NLR influences survival in esophageal cancer remains unclear. Tumors consist of not only neoplastic cells but also a microenvironment. Infiltrating leukocytes and stromal cells make up the microenvironment wherein they engage in continuous interactions with both tumor cells and each other (33). Increasing studies have found that neutrophils have the ability to synthesize a series of cytokines with multifaceted effects, including tumor promotion, angiogenesis, and progression (34,35). One example is APRIL, a neutrophil-derived cytokine involved in tumor progression reported to be broadly expressed in normal tissue, tumor cells, and peripheral blood (36). Lymphocytes in the peripheral blood are currently thought to cause synergistic cytotoxicity and exert tumor suppressor properties (37). The NLR, a useful biomarker that can be calculated quite easily from routine blood cell tests, may reflect the balance between the complex systemic inflammation.
Both pre- and post-NLR were not significantly associated with frequency of leukopenia and neutropenia during chemotherapy. On the other hand, the high groups of both pre- and post-NLR had significantly more incidences of lymphopenia compared to the low groups. Although no change in lymphocyte count had apparently been observed before and after chemotherapy, a dramatical change in lymphocyte count had in fact occurred during chemotherapy, which may have strongly influenced the NLR. Additionally, the high pre-NLR group had significantly more incidences of anemia than the low pre-NLR group. The high pre-NLR had significantly more patients with locally advanced tumor (cT3 or T4) (76 out of 87, 87%) than low pre-NLR (176 out of 251, 70%) (P=0.002). The chronic inflammation or bleeding caused by the advanced tumor may consequently cause frequent anemia in patients with high pre-NLR.
The present study observed differences in the NLR and neutrophil counts before and after NAC. Furthermore, the NLR after NAC had been determined to be a better prognostic factor than that before NAC. Our multivariate analysis for post-NLR identified lymphopenia, as well as higher neutrocyte counts and lower lymphocyte counts, as an independent factor for high post-NLR. Patients who exhibit sensitivity to ACF or DCF have dramatically reduced tumor volumes (16). The present study showed that patients with high post-NLR had significantly worse clinical responses than those with low post-NLR. Chemo-resistant patients with a high post-NLR still have numerous tumor cells remaining after chemotherapy. The tumor cells and surrounding microenvironment promote a systemic inflammatory response (9), which can be reflected in circulating neutrophil and lymphocyte counts. This suggests that a higher NLR after NAC may imply resistance to treatment, which can correlate with prognosis.
Interestingly, multivariate analysis identified lower BMI as an independent factor for high post-NLR, indicating a possible association between NLR and nutritional status. A few patients with esophageal cancer present with malnutrition upon initial diagnosis. Hagi et al reported that 92 out of 434 patients with esophageal cancer were able to swallow only liquids or had complete dysphagia (38). Patients suffering from dysphagia require intensive support mainly via enteral nutrition during chemotherapy, which may consequently suppress systemic inflammation. Improvement in a patients' nutritional status during NAC may help correct the NLR, consequently making NLR after NAC a more accurate prognostic factor than that before NAC. Conversely, the nutritional intervention itself during chemotherapy might suppress the cascade of immune response, which contributes to better prognosis.
Some limitations of the current study are worth noting. The present study carried a retrospective design and was conducted at a single institution. Furthermore, nutritional parameters had not been assessed herein. This is significant given Baker's report suggesting that elevated NLR was associated with greater weight loss and cachexia in patients with advanced colon, lung, or prostate cancer (39). Considering that 21% of patients with esophageal cancer suffer from dysphagia (38), future studies should evaluate the association between nutrition and inflammation.
In conclusion, the current study showed that NLR after NAC was a better prognostic factor than that before NAC for patients receiving triplet chemotherapy followed by surgical resection.
Supplementary Material
Acknowledgements
The authors acknowledge Dr S. Komukai (Division of Biomedical Statistics, Department of Integrated Medicine, Graduate School of Medicine, Osaka University) for his advice.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MoY and KT conceived and designed the present study. MoY, KT, MaY, KY, TM, TS, TT, YKu, MM, YKi, KN, HE and YD contributed to data acquisition and analysis. MoY and KT were major contributors in writing the manuscript and confirmed the authenticity of all the raw data. All authors provided supervision of the manuscript, and read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Human Ethics Review Committee of the Osaka University School of Medicine (approval no. 16305-4) and written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Medical Research Council Oesophageal Cancer Working Group Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 3.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 4.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, Tamura S, Kimura Y, Miyata H, Motoori M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003) Ann Oncol. 2017;28:116–120. doi: 10.1093/annonc/mdw439. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi O, Yamasaki M, Makino T, Motoori M, Miyata H, Shinkai M, Kimura Y, Hirao M, Fujitani K, Tamura S, et al. Feasibility of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil versus adriamycin, cisplatin, and 5-fluorouracil for resectable advanced esophageal cancer. Oncology. 2017;92:101–108. doi: 10.1159/000452765. [DOI] [PubMed] [Google Scholar]
- 7.Shimakawa T, Naritaka Y, Asaka S, Isohata N, Murayama M, Konno S, Yoshimatsu K, Shiozawa S, Katsube T, Ogawa K. Neoadjuvant chemotherapy (FAP) for advanced esophageal cancer. Anticancer Res. 2008;28:2321–2326. [PubMed] [Google Scholar]
- 8.Motoori M, Yano M, Yasuda T, Miyata H, Peng YF, Yamasaki M, Shiraishi O, Masuzawa Y, Tanaka K, Ishikawa O, et al. Chemotherapy-induced toxicities and treatment efficacy in advanced esophageal cancer treated with neoadjuvant chemotherapy followed by surgery. Esophagus. 2011;8:81–87. doi: 10.1007/s10388-011-0267-7. [DOI] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol Med. 2018;15:88–96. doi: 10.20892/j.issn.2095-3941.2017.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Jiang C, Li J, Sun J, Qu X. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in Patients with gallbladder carcinoma. Clin Transl Oncol. 2015;17:810–818. doi: 10.1007/s12094-015-1310-2. [DOI] [PubMed] [Google Scholar]
- 13.Huszno J, Kolosza Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol Lett. 2019;18:6275–6283. doi: 10.3892/ol.2019.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil: Lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–3369. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zeng H, Yang J, Lu Y, Zhang D, Wang J, Kuang C, Zhu S, Wang M, Ma X. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018;18:816. doi: 10.1186/s12885-018-4730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasaki M, Miyata H, Tanaka K, Shiraishi O, Motoori M, Peng YF, Yasuda T, Yano M, Shiozaki H, Mori M, Doki Y. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307–313. doi: 10.1159/000329806. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino S, Takeuchi M, Kawakubo H, Kobayashi R, Matsuda S, Irino T, Fukuda K, Nakamura R, Kitagawa Y. Neutrophil-to-lymphocyte ratio change predicts histological response to and oncological outcome of neoadjuvant chemotherapy for esophageal squamous cell carcinoma. Esophagus. 2022;19:426–435. doi: 10.1007/s10388-021-00901-6. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th edition. New York: Wiley-Blackwell; 2009. UICC International Union Against Cancer. [Google Scholar]
- 20.Japan Esophageal Society. Japanese classification of esophageal cancer, 11th edition: Part II and III. Esophagus. 2017;14:37–65. doi: 10.1007/s10388-016-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute, corp-author. Common terminology criteria for adverse events (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 [Google Scholar]
- 22.Yano M, Takachi K, Doki Y, Miyashiro I, Kishi K, Noura S, Eguchi H, Yamada T, Ohue M, Ohigashi H, et al. Preoperative chemotherapy for clinically node-positive patients with squamous cell carcinoma of the esophagus. Dis Esophagus. 2006;19:158–163. doi: 10.1111/j.1442-2050.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus. 2019;16:25–43. doi: 10.1007/s10388-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita K, Makino T, Yamasaki M, Tanaka K, Hara T, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, et al. Comparison of short-term outcomes between 2- and 3-field lymph node dissection for esophageal cancer. Dis Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox096. [DOI] [PubMed] [Google Scholar]
- 25.Toh Y, Kitagawa Y, Kuwano H, Kusano M, Oyama T, Muto M, Kato H, Takeuchi H, Doki Y, Naomoto Y, et al. A nation-wide survey of follow-up strategies for esophageal cancer patients after a curative esophagectomy or a complete Response by definitive chemoradiotherapy in Japan. Esophagus. 2016;13:173–181. doi: 10.1007/s10388-015-0511-7. [DOI] [Google Scholar]
- 26.Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 27.Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: A systematic review and meta-analysis. J Thorac Dis. 2019;11:3136–3145. doi: 10.21037/jtd.2019.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki T, Sakai M, Sohda M, Tanaka N, Yokobori T, Motegi Y, Nakajima M, Fukuchi M, Kato H, Kuwano H. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res. 2016;36:6557–6562. doi: 10.21873/anticanres.11259. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143:2077–2086. doi: 10.1007/s00432-017-2451-1. [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi Y, Tsujimoto H, Hiraki S, Kumano I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018;25:3288–3299. doi: 10.1245/s10434-018-6651-y. [DOI] [PubMed] [Google Scholar]
- 31.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–2016. doi: 10.1007/s00432-012-1281-4. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z. Inflammatory cells and cancer: Think different! J Exp Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassatella MA. Neutrophil-derived proteins: Selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/S0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 35.Scapini P, Bazzoni F, Cassatella MA. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett. 2008;116:1–6. doi: 10.1016/j.imlet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Jabłońska E, Wawrusiewicz-Kurylonek N, Garley M, Ratajczak-Wrona W, Antonowicz B, Dziemiańczyk-Pakieła D, Jabłoński J, Krętowski A, Grabowska SZ. A proliferation-inducing ligand (APRIL) in neutrophils of patients with oral cavity squamous cell carcinoma. Eur Cytokine Netw. 2012;23:93–100. doi: 10.1684/ecn.2012.0311. [DOI] [PubMed] [Google Scholar]
- 37.Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, Hayashi N, Yokoyama K, Oshitanai R, Terao M, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107:1730–1735. doi: 10.1111/cas.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagi T, Makino T, Yamasaki M, Tanaka K, Nishida N, Sakai D, Motoori M, Kimura Y, Satoh T, Mori M, Doki Y. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26:4754–4764. doi: 10.1245/s10434-019-07744-7. [DOI] [PubMed] [Google Scholar]
- 39.Barker T, Fulde G, Moulton B, Nadauld LD, Rhodes T. An elevated neutrophil-to-lymphocyte ratio associates with weight loss and cachexia in cancer. Sci Rep. 2020;10:7535. doi: 10.1038/s41598-020-64282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.