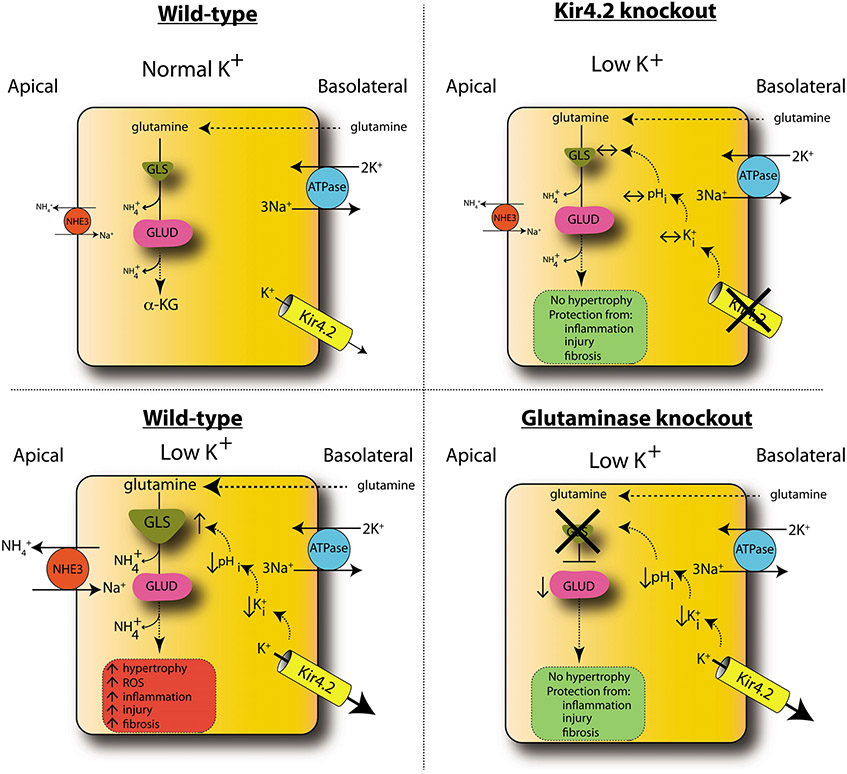
Figure 7. Model of low-K+ kidney injury.
In WT animals, low K+ causes increased basolateral K+ efflux through Kir4.2, reducing intracellular K+ (K+i) and pHi. This increases glutamine uptake along the basolateral membrane and its catabolism through GLS, producing ammonia, which is secreted into the lumen via NHE3. Glutamate dehudrogenase (GLUD) catalyzes conversion to α-ketoglutarate (α-KG). Metabolic flux through this pathway is increased under low-K+ conditions, causing increased kidney hypertrophy, inflammation, injury, and fibrosis. Kir4.2 deletion prevents alterations in basolateral K+ flux under low-K+ conditions. Kir4.2−/− mice do not develop intracellular acidosis or increased glutamine catabolism and are protected from kidney hypertrophy and injury. While GLS knockout mice do develop an intracellular acidosis under low-K+ conditions, the absence of GLS prevents increased glutamine catabolism, affording renoprotection. Error bars indicate SEM.