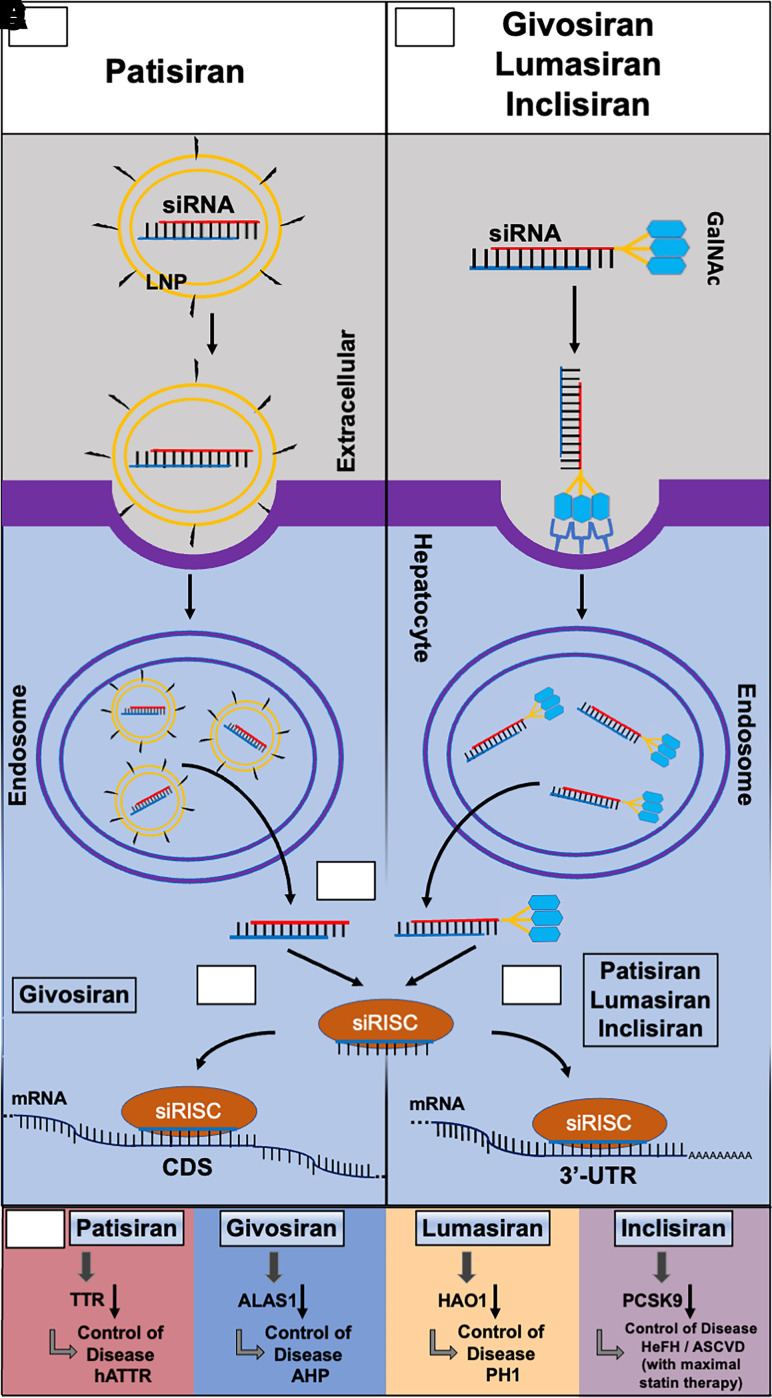
Fig. 2.
Common and specific actions of four FDA-approved siRNA medications in hepatocytes. (A) Patisiran is a double stranded siRNA drug (sense in red and antisense in blue) formulated in a lipid nanoparticle (LNP) decorated with polyethylene glycol (pegylation), and it is administered intravenously for the treatment of hereditary transthyretin-mediated amyloidosis. The LNP induces an opsonization-based immune response and is endocytosed by the hepatocyte prior to endosomal escape. (B) Givosiran, lumasiran, and inclisiran, in which the sense strand (red) is conjugated with three N-acetylgalactosamine (GalNAc) moieties, are administered subcutaneously for the treatments of acute hepatic porphyria, primary hyperoxaluria type 1, and heterozygous familial hypercholesterolemia, respectively. The GalNAc moieties are recognized by asialoglycoprotein receptor 1 (ASGR1), which is highly expressed on the surface of hepatocytes, to facilitate the uptake of siRNAs. (C) The antisense strand (blue) is preferably loaded into the RNA-induced silencing complex (RISC) to form the siRNA-RISC complex (siRISC) while the passenger strand (red) is degraded. (D) Givosiran-derived siRISC binds to the protein coding sequence (CDS) of target mRNA toward cleavage or degradation. (E) Patisiran-, lumasiran-, and inclisiran-derived siRISC interacts with the 3′-untranslated regions (3′UTRs) of target mRNAs to achieve gene silencing. (F) SiRNA drugs (givosiran, patisiran, lumasiran, and inclisiran) target specific mRNAs to achieve the control of their respective diseases. HAO1, hydroxyacid oxidase 1.