Abstract
Aims.
A randomized control trial (RCT) of diabetes self-management education (DSME), undertaken by a community-based participatory research (CBPR) partnership between the University of Arkansas for Medical Sciences (UAMS) and the Marshallese community in Arkansas. The RCT examined the effect of the hours of intervention exposure, with the hypothesis that increased exposure is one reason the Adapted-Family DSME was found to be more effective than the Standard DSME.
Methods.
221 Marshallese with type 2 diabetes were randomized to an Adapted-Family DSME group (in-home setting) (n=110) or a Standard DMSE group (community setting) (n=111). The Adapted-Family DSME included 10 hours of education that covered the core self-care elements recommended by the American Diabetes Association (ADA) and American Association of Diabetes Educators’ (AADE) recommendations. The Standard DSME included 10 hours of intervention with all ADA and AADE core elements.
Results.
The number of hours of intervention exposure in the Adapted-Family DSME arm (Mean=8.0; Median=10.0) was significantly higher than the number of hours of intervention received in the Standard DSME arm (Mean=1.5; Median=0.0). As hypothesized, higher exposure was associated with a significant reduction in HbA1c in a model including only study arm and exposure (P=0.01), and in a model including study arm, exposure, and all demographic variables (P=0.046).
Conclusions.
This finding is consistent with previous reviews that showed increased exposure to DSME produced improved glycaemic control and 10 hours or more of DSME produces clinically meaningful reductions in HbA1c.
Keywords: type 2 diabetes, diabetes self-management education, adapted-family model, randomized controlled trial, comparative effectiveness, Pacific Islanders, Marshallese
INTRODUCTION
The Pacific Islander population in the US is small but rapidly growing, with a 40% increase between 2000 and 2010 across the country, and a more than 250% increase in the state of Arkansas [1]. Most Pacific Islanders in Arkansas are Marshallese, and Arkansas has the largest Marshallese population in the continental US with around 10,000 residents [2]. Marshallese experience high incidence of type 2 diabetes, with estimates among adults living in the Republic of the Marshall Islands (RMI) and the US ranging from 20% to 40% [3, 4]. Specifically in northwest Arkansas, screening data (n=401) has documented high incidence of type 2 diabetes (38.4%) and pre-diabetes (32.6%) among Marshallese [5].
To understand the diabetes epidemic among the Marshallese, it is important to understand the historical relationship between the RMI and the US. Between 1946 and 1958, the US tested nuclear weapons in the RMI. Tests exposed islanders to significant levels of nuclear radiation [6]. The nuclear testing disrupted the food supply through the contamination of fish and plants on the islands. The islanders who had previously consumed fresh fish and local vegetables, transitioned to a diet of highly processed, canned foods high in refined carbohydrates and fat that were shipped in by the US [7].
After the nuclear tests, US scientists set up Project 4.1 to study the effects of nuclear radiation on humans [6]. The research was conducted without informed consent and without language translation. Subsequent studies demonstrated health problems affecting those exposed and changes in Marshallese health behaviours [6–8]. Like other cultures marked by historical trauma, the Marshallese exhibit distrust in researchers [9]. The research team overcame this trauma and distrust through the use of a community-based participatory research (CBPR) approach. The present research is a follow up to a randomized control trial (RCT) of diabetes self-management education (DSME), undertaken by a CBPR partnership between the University of Arkansas for Medical Sciences (UAMS) and the Marshallese community in Arkansas [10–13].
DSME has been shown to be effective at improving diabetes-related clinical outcomes, including glycaemic control [14, 15]. There have been studies of DSME among Marshallese in Hawaii and the RMI; however, the two prior studies did not demonstrate improved glycaemic control [16, 17]. Other studies suggest that culturally appropriate DSME is more effective in minority and immigrant communities [18, 19], and family models of DSME are effective in minority communities [20, 21]. However, prior to our study, no known culturally-adapted or family models of DSME have been tested with Marshallese participants. Our study compared the effectiveness of a Standard DSME curriculum to that of our Adapted-Family DSME curriculum. The primary study outcomes paper presented significantly greater declines in adjusted mean HbA1c achieved in the Adapted-Family DSME arm compared with the Standard DSME arm immediately after (Adj. Mean Diff.=−0.61, P=0.04) [13].
The number of hours of DSME intervention patients receive has been shown to be an important factor in reduction of HbA1c. In a meta-analysis of RCTs, total contact hours was positively associated with the effect of DSME on improved glycaemic control [22]. Similarly, a systematic review showed that receiving 10 hours or more of DSME intervention increased the likelihood of a significant reduction in HbA1c [23].
The objective of this article is to present results of a post-trial analysis to evaluate the DSME exposure (hours of attendance) participants received, with the hypothesis that increased exposure is one of the possible reasons the Adapted-Family model of DSME was found to be more effective than the Standard DSME.
RESEARCH DESIGN AND METHODS
Bilingual Marshallese staff recruited potential participants through community/church health screenings [5], self-referrals from members of the community who had heard about the study, local community health worker referrals, community partners, and local clinics which serve Marshallese patients [13, 24]. Participants were Marshallese adults (aged 18 and older) who had type 2 diabetes. Those meeting the inclusion criteria were provided information about the study in English or Marshallese and were given the opportunity to discuss the study with bilingual Marshallese research staff. The study was approved by the UAMS Institutional Review Board (#203482) and registered at clinicaltrials.gov (#NCT02407132) and HSRProj (#HSRP20152031). The study design utilized was a community-based participatory research approach. A detailed protocol has been published elsewhere [24, 25].
The study took place in Washington and Benton Counties, located in northwest Arkansas, where 240 participants agreed to participate in the study and provided consent. Each participants’ type 2 diabetes status was confirmed using baseline HbA1c, occurring after enrolment but before implementation of the two interventions. Participants not on glucose-lowering medications who did not have HbA1c results indicating diabetes (6.5% or above) were unenrolled from the study at baseline data collection. Eligible participants were randomly assigned to either the Adapted-Family DSME arm or the Standard DSME arm. Randomization was conducted at the family level. Prior to this, consented participants were grouped by family, preventing members from the same family from being assigned to different arms (i.e., to minimize cross-contamination). Each family was randomly assigned to one of the two study arms. Randomization was conducted using a random number generation function, which concealed the families’ identities from the person who made the assignment. The investigator who conducted randomization had no interactions with potential participants and no supervisory role with program staff responsible for recruitment, consent, or intervention delivery. The target sample size of 240 allowed us to achieve 80% power to detect an effect size of d=0.3 in a design with four time points of measurement and a compound symmetry covariance structure, with a correlation of 0.5 between observations on the same subject and an alpha level of 0.05 assumed [26]. Consistent across both arms of the study, retention efforts included bi-weekly participant contact via e-mail and phone. A $20 gift card was provided as an incentive at each of the four data collection events.
Intervention Description
The Standard DSME provided a total of 10 hours of education, covering eight core elements across sessions: healthy eating, being active, glucose monitoring, understanding blood glucose and taking medications, problem solving, reducing risks and healthy coping, mitigating complications of diabetes, and goal setting. The core elements were consistent with the American Diabetes Association (ADA) and American Association of Diabetes Educators’ (AADE) recommendations regarding self-care behaviours [27]. Family members of participants in the Standard DSME arm were not invited to the education sessions.
The Adapted-Family DSME also provided 10 total hours and covered the same eight core elements of the Standard DSME. The curriculum, however, was culturally adapted using a CBPR approach. Curriculum changes included: culturally appropriate nature analogies (e.g., tide changes) to assist in the explanation of changes in glucose numbers; discussions of the importance of medication adherence, focusing on natural plant-based properties of metformin; and emphasizing engagement of participants’ family orientation as a means of self-management. Specifically, the curriculum focused heavily on the importance of engaging all family members in desired behavioural changes and incorporated goal setting and motivational interviewing at the family level. Participants were encouraged to invite family members to each session, and the Adapted-Family DSME curriculum was based on active engagement of family members in each educational intervention.
The Adapted-Family DSME was conducted in participants’ homes. The Standard DSME was conducted at a local non-profit that was well-known to participants and was located in close proximity to the Marshallese community. The intervention was implemented between 2015 and 2018. A detailed description of each intervention is published elsewhere [13].
Study Outcomes
Change in mean HbA1c from baseline to immediate post-intervention (9 weeks) was the primary outcome of interest. HbA1c was assessed through finger prick blood collection using a Rapid A1c test kit and Siemens DCA Vantage Analyzer. Control for use of medication prescribed to lower blood glucose was incorporated into each analysis of the study. These medications were coded as Yes/No based upon participant’s self-reported use.
Analytical and Statistical Approaches
The study design was a two-arm comparative effectiveness RCT. We fitted three, progressive linear mixed effects regression models for repeated measures to analyse the impact of DSME exposure (hours of classes attended) on the difference in the change in HbA1c from baseline to immediate post-intervention between Adapted-Family DSME and Standard DSME participants. Each model was adjusted for baseline HbA1c before entering additional variables. Any familial correlation was incorporated as a random effect in the models, with the assumption of compound symmetry as the underlying covariance structure.
The initial model included only study arm assignment. The second model added exposure (hours of attendance) to model 1. The final model included the variables from models 1 and 2, adjusting for sex, age, education, marital status, employment status, and use of diabetes medication. All analyses were carried out using SAS/STATv14.2. Statistical significance was set at the a priori alpha level of 0.05.
The intent-to-treat principal was the foundation of the analyses of our primary outcome variable (change in mean HbA1c). This ensured that regardless of the compliance with the protocol of the study or non-completion of the study, data from all randomized participants were analysed.
Participant Flow
Of the 240 participants consented to be screened, 221 were eligible and randomized to one of the two study arms. The study’s CONSORT Flow Diagram is presented in Fig. 1.
Figure 1.
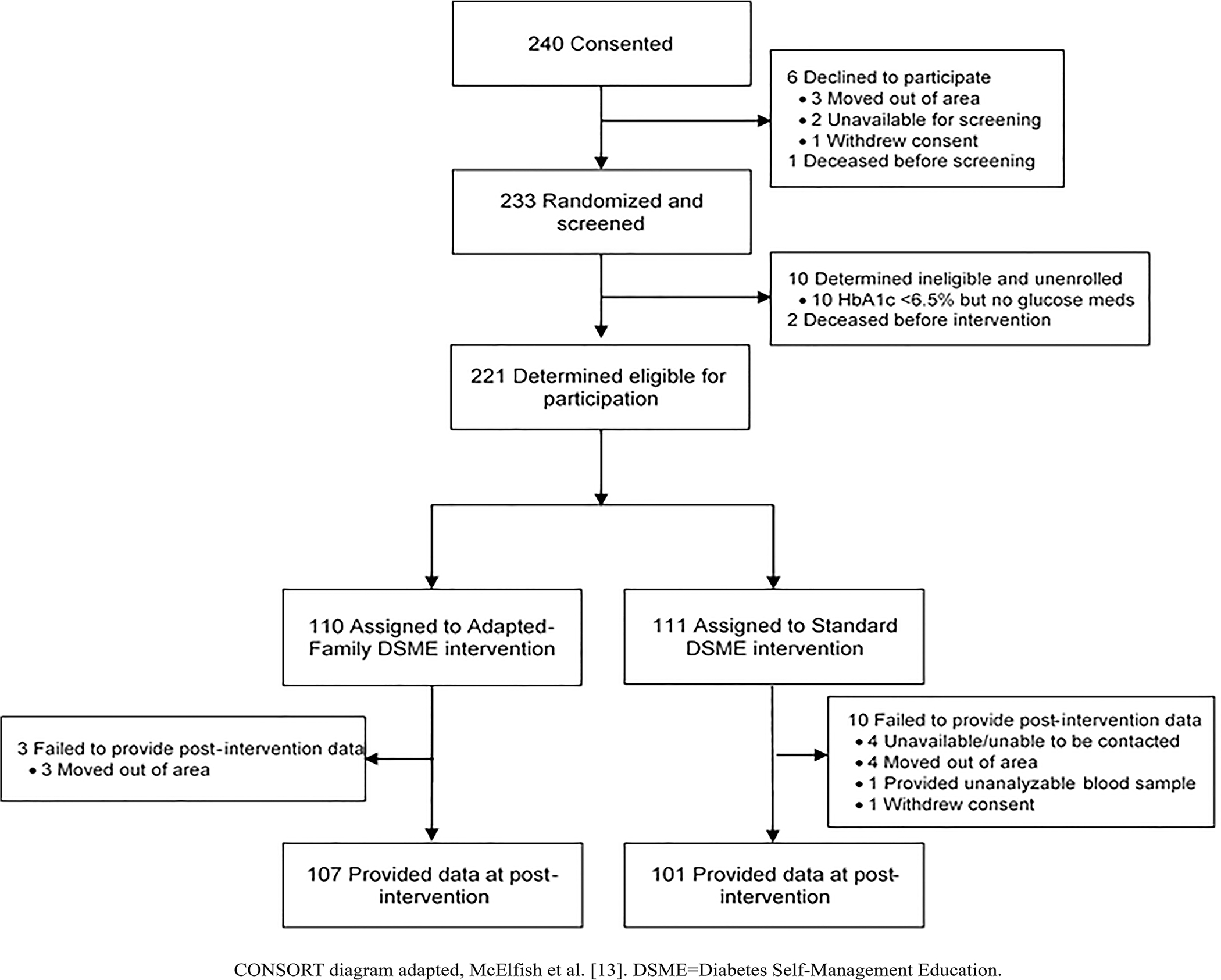
Enrollment, randomization, and retention of study participants.
RESULTS
Characteristics of Participants
The baseline characteristics of study participants by arm are shown in Table 1. Across all 221 participants, the median age was 52 years (range=31 to 80 years), and less than half were men (42.2%). Retention of participants was not significantly different between arms. At immediate post-intervention, 91.0% of Standard DSME participants and 97.3% of Adapted-Family DSME participants provided outcome data (P=0.150).
Table 1.
Baseline characteristics of participants by study arm
| Adapted-Family DSME (N=110) | Standard DSME (N=111) | |
|---|---|---|
|
| ||
| Baseline HbA1c; mmol/mol (%) * | 91 ± 26 (10.5 ± 2.4) | 90 ± 24 (10.4 ± 2.2) |
| Age † | 51.5 (31–80) | 52 (33–79) |
| Male | 52 (47) | 39 (35) |
| Married | 82 (75) | 78 (70) |
| Education | ||
| Less than HS | 66 (60) | 69 (62) |
| HS/GED | 30 (27) | 34 (31) |
| Higher than HS | 14 (13) | 8 (7.3) |
| Employed | 47 (43) | 38 (34) |
| Any diabetes medication | 48 (44) | 49 (44) |
Values are given as n (%) unless otherwise indicated
mean ± SD
median (range).
HbA1c, glycated haemoglobin; DSME, diabetes self-management education; HS, high school; Any diabetes medication, participant was taking at least one medication to regulate blood glucose.
Changes in Outcome
Table 2 provides the summary statistics for attendance hours of the total sample and the two study arms. The hours of intervention received varied by study arm, with Standard DSME participants receiving a mean of 1.5 hours of intervention, and Adapted-Family DSME participants receiving a mean of 8.0 hours of intervention.
Table 2.
Summary statistics for exposure (hours of attendance) overall, by study arm
| Study Arm Assignment | N | Mean | SD | Mdn | Min | Max |
|---|---|---|---|---|---|---|
|
| ||||||
| All Participants | 221 | 4.8 | 4.5 | 3.8 | 0.0 | 10.0 |
| Standard | 111 | 1.5 | 3.0 | 0.0 | 0.0 | 10.0 |
| Adapted-Family | 110 | 8.0 | 3.2 | 10.0 | 0.0 | 10.0 |
SD=standard deviation; Mdn=Median; Min=Minimum; Max=Maximum
The distribution of intervention hours by study arm (Fig. 2) shows 70.3% of Standard DSME arm participants were exposed to 0 hours of DSME, while 54.6% of Adapted-Family DSME arm participants were exposed to 10 hours. A Wilcoxon Rank Sum test revealed significant distributional differences for the hours of intervention between the Adapted-Family arm and Standard arm participants (P<0.01).
Figure 2.

Distribution of exposure (hours of attendance) by study arm.
In Table 3, the first mixed effects model shows a significant effect of study arm on reduction of HbA1c, where Adapted-Family DSME was associated with greater reductions than the Standard DSME (P=0.029). In the second model, incorporation of exposure (hours of attendance) confounds the effect of study arm on reduction of HbA1c, which becomes statistically non-significant (P=0.633); however, exposure is significant (P=0.013). The final model incorporated adjustments for sex, age, education, marital status, employment status, and use of diabetes medication, which did not affect the previous study arm difference (P=0.655), and the effect of exposure remained significant (P=0.046).
Table 3.
Mixed effects regression models estimating associations between intervention and participation on change in HbA1c
| Change in HbA1c (mmol/mol) | |||||||||
| Model 1 † | Model 2 † | Model 3 † | |||||||
|
| |||||||||
| Effect | B(95%CI) | SE | P | B(95% CI) | SE | P | B(95% CI) | SE | P |
|
| |||||||||
| Adapted-Family | −5 (−10, −1) | 2.3 | 0.029 | 2 (−5, 9) | 3.5 | 0.633 | −2 (−8, 5) | 3.4 | 0.655 |
| Exposure | −1 (−2, 0) | 0.4 | 0.013 | −1 (−1, 0) | 0.3 | 0.046 | |||
| Age | −0 (0, 0) | 0.1 | 0.254 | ||||||
| Male | 1 (−4, 6) | 2.5 | 0.645 | ||||||
| Married | 0 (−5, 5) | 2.6 | 0.995 | ||||||
| Employed | 0 (−5, 5) | 2.6 | 0.953 | ||||||
| Education | 1 (−2, 3) | 1.6 | 0.376 | ||||||
| Medication | −3 (−7, 2) | 2.4 | 0.279 | ||||||
|
| |||||||||
| Change in HbA1c (%) | |||||||||
| Model 1 † | Model 2 † | Model 3 † | |||||||
|
| |||||||||
| Effect | B(95%CI) | SE | P | B(95% CI) | SE | P | B(95% CI) | SE | P |
|
| |||||||||
| Adapted-Family | −0.5 (−0.9, −0.1) | 0.21 | 0.029 | 0.2 (−0.5, 0.8) | 0.32 | 0.633 | −0.1 (−0.8, 0.5) | 0.31 | 0.655 |
| Exposure | −0.1 (−0.2, 0.0) | 0.04 | 0.013 | −0.1 (−0.1, 0.0) | 0.03 | 0.046 | |||
| Age | −0.0 (−0.0, 0.0) | 0.01 | 0.254 | ||||||
| Male | 0.1 (−0.3, 0.6) | 0.23 | 0.645 | ||||||
| Married | 0.0 (−0.5, 0.5) | 0.24 | 0.995 | ||||||
| Employed | 0.0 (−0.5, 0.5) | 0.24 | 0.953 | ||||||
| Education | 0.1 (−0.2, 0.3) | 0.15 | 0.376 | ||||||
| Medication | −0.2 (−0.7, 0.2) | 0.22 | 0.279 | ||||||
Statistically significant P values are bolded (p<0.05). DSME, diabetes self-management education; HbA1c, glycated haemoglobin; SE=standard error.
Adjusted for baseline HbA1c.
DISCUSSION
This article evaluated the effect of participants’ intervention exposure (hours of attendance) in an RCT comparing an Adapted-Family DSME with a Standard DSME. We hypothesized that increased exposure is one of the possible reasons Adapted-Family DSME was found to be more effective in reducing HbA1c than Standard DSME. While both arms included a 10-hour intervention, participants’ attendance varied greatly between the study arms: the number of hours of intervention exposure in the Adapted-Family DSME arm (Mean=8.0; Median=10.0) was significantly higher than the number of hours of intervention received in the Standard DSME arm (Mean=1.5; Median=0.0). As hypothesized, the higher exposure was associated with a significant reduction in HbA1c. This finding is consistent with previous reviews that showed increased exposure to DSME produced improved glycaemic control [22, 23], and 10 hours or more of DSME produces clinically meaningful reductions in HbA1c [28, 29].
There could be several reasons for the difference in intervention exposure between the study arms. The Adapted-Family DSME curriculum’s cultural resonance could have encouraged participants to attend the intervention. The Adapted-Family model was developed using an intensive CBPR process [11], and was adapted to include culturally appropriate components throughout the DSME curriculum. The increased exposure could also be because the intervention engaged the participants’ family, and the engagement of family members in DSME improved participation. The higher exposure could also be because the Adapted-Family DSME was delivered in participants’ homes which removed common barriers to DSME attendance such as transportation, making it easier for participants to attend the intervention and receive increased exposure.
We might speculate that it is the exposure to either intervention and not the differences in intervention content that are responsible for differences in glycaemic control. However, we cannot separate the two; exposure appears to be a function of intervention. The magnitude of the effect of the Standard DSME intervention with more participation is unknown. The lack of variation in attendance within arms suggests that the Adapted-Family DSME may have improved attendance and, therefore, exposure to the intervention. The increased exposure and effectiveness of the Adapted-Family DSME are consistent with prior literature that shows culturally-adapted models of DSME are often more effective with minority and immigrant populations [18, 19], and that DSME delivered in the home is effective in improving glycaemic control [30]. Interestingly, retention in the study’s data collection events were not significantly different, suggesting that retention efforts across arms were consistent and differences in exposure were due to the characteristics of the interventions.
Limitations
The study results should be examined in light of some limitations. All participants in this study were Marshallese adults living in Arkansas, which limits the generalizability of the results to other Pacific Islander populations or Marshallese living outside Arkansas. Despite this limitation, this study adds much to the literature. This study replicated the finding that increased exposure to DSME is a mechanism significantly associated with improved glycaemic control. However, the Adapted-Family DSME adapted multiple interventions components specifically designed to overcome documented challenges faced by the population (e.g., culturally relevant curriculum, delivered in the home, by a community health worker) at one time [8, 24, 25]. We do not know if just one of the adaptations or the simultaneous implementation of multiple adaptations were responsible for increased exposure and the observed results.
Furthermore, delivering a home-based intervention was very time consuming, and a cost analysis was not completed which limits are understanding of the cost-benefit of the Adapted-Family DSME. Future studies should examine the effect of each of the components individually and their influence on increasing exposure and on HbA1c. Future studies should also include cost-effectiveness analysis. These limitations notwithstanding, the Adapted-Family DSME resulted in increased exposure, which is associated with increased effectiveness. Future research should examine the extent to which the Adapted-Family DSME may be effective in increasing exposure and effectiveness in other groups, including other minority and immigrant populations.
Novelty Statement.
Use of culturally adapted diabetes self-management education has been shown to be effective in improving levels of glycated haemoglobin.
The exposure to education explains differences in improvement of glycated haemoglobin levels.
Exposure to 10 hours or more of diabetes self-management education is necessary to observe the desired improvement in glycated haemoglobin levels.
Acknowledgements:
The authors thank members of the research team and community advisory board that are not authors, but whose work contributed to the success of the study: Lisa Smith, Gwen Wiley, Nia Aitaoto, Wana Bing, Kejjo Clarence, Melisa Laelan, Krista Langston, Morda Netwon, Mandy Ritok-Lakien, Tori Rowe, Jellesen Rubon-Chutaro, Karra Sparks, Melissa Wann, Ralph Wilmoth, Tracy Canant, Erica Powell, Melissa Brown, Charles Heam, Sandy Joel, Sadie Briand Kabua, Primrose Jones, Sheldon Riklon, Jonell Hudson, and Joe Kaminaga.
Funding.
Financial support for the study was provided by a grant from the Patient-Centered Outcomes Research Institute (#AD-1310–07159). Initial funding for a pilot project of the study was provided by a University of Arkansas for Medical Sciences College of Medicine Intramural Sturgis Grant for Diabetes Research from the Sturgis Foundation. Additional support for the community-based participatory research team was provided by a Translational Research Institute grant (#1U54TR001629–01A1) from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Conflict of Interest Disclosure: The authors have no conflicts of interest to report.
REFERENCES
- 1.Hixson L, Hepler B, Kim M. The Native Hawaiian and Other Pacific Islander population 2010. Washington, DC: United States Census Bureau; 2012. [Google Scholar]
- 2.United States Census Bureau. American Community Survey Demographic and Housing Estimates: 2012–2016 ACS 5-year estimates, Table DP05 2016. [Available from: factfinder.census.gov.
- 3.Yamada S, Dodd A, Soe T, Chen T, Bauman K. Diabetes mellitus prevalence in out-patient Marshallese adults on Ebeye Island, Republic of the Marshall Islands. Hawaii Medical Journal [Internet]. 2004; 63(2):[45–51 pp.]. [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas, 8th edition. Brussels, Belguim: International Diabetes Federation; 2017. [Google Scholar]
- 5.McElfish P, Rowland B, Long C, Hudson J, Piel M, Buron B, et al. Diabetes and hypertension in Marshallese adults: Results from faith-based health screenings. J Racial Ethn Health Disparities. 2017;4(6):1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker H Bravo for the Marshallese: Regaining Control in a Post-Nuclear, Post-Colonial World. Belmont, CA: Cengage Learning; 2012. [Google Scholar]
- 7.Gittelsohn J, Haberle H, Vastine A, Dyckman W, Palafox N. Macro- and microlevel processes affect food choice and nutritional status in the Republic of the Marshall Islands. The Journal Of Nutrition. 2003;133(1):310S–3S. [DOI] [PubMed] [Google Scholar]
- 8.McElfish P, Hallgren E, Henry L, Ritok M, Rubon-Chutaro J, Kohler P. Health beliefs of Marshallese regarding type 2 diabetes. American Journal of Health Behavior. 2016;40(1):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran E, Duran B, Yellow Horse Brave Heart M, Yellow Horse-Davis S. Healing the American Indian Soul Wound. In: Danieli Y, editor. International Handbook of Multigenerational Legacies of Trauma. New York: Springer; 1998. p. 341–54. [Google Scholar]
- 10.McElfish P, Long C, Stephens R, Spencer N, Rowland B, Spencer H, et al. Assessing community health priorities and perceptions about health research: a foundation for a community-engaged research program. J High Educ Outreach Engagem. 2018;22(1):107–28. [Google Scholar]
- 11.McElfish PA, Goulden PA, Bursac Z, Hudson J, Purvis RS, Kim Yeary KH, et al. Engagement practices that join scientific methods with community wisdom: designing a patient-centered, randomized control trial with a Pacific Islander community. Nurs Inq. 2017;24(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElfish P, Kohler P, Smith C, Warmack S, Buron B, Hudson J, et al. Community-driven research agenda to reduce health disparities. Clin Transl Sci. 2015;8(6):690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElfish P, Long C, Kohler P, Yeary K, Bursac Z, Narcisse M, et al. Comparative effectiveness and maintenance of diabetes self-management education interventions for Marshallese type 2 diabetes patients: a randomized controlled trial. Diabetes Care. 2019;42:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan C, Lai CW, Chan LW, Chow M, Law HK, Ying M. The effect of diabetes self-management education on body weight, glycemic control, and other metabolic markers in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:789761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunisholz KD, Briot P, Hamilton S, Joy EA, Lomax M, Barton N, et al. Diabetes self-management education improves quality of care and clinical outcomes determined by a diabetes bundle measure. J Multidiscip Healthc. 2014;7:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy R, Shehata C, Smith G, Maskarinec G. Characteristics of Marshallese with Type 2 Diabetes on Oahu: A Pilot Study to Implement a Community-Based Diabetic Health Improvement Project. Californian Journal of Health Promotion. 2005;3(4):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy R, Trinidad R, Seremai J, Nasa J. Marshallese diabetic health improvement pilot project in Ebeye. Californian Journal of Health Promotion. 2009;7:125–30. [Google Scholar]
- 18.Creamer J, Attridge M, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for Type 2 diabetes in ethnic minority groups: an updated Cochrane Review of randomized controlled trials. Diabet Med. 2016;33(2):169–83. [DOI] [PubMed] [Google Scholar]
- 19.Ricci-Cabello I, Ruiz-Perez I, Rojas-Garcia A, Pastor G, Rodriguez-Barranco M, Goncalves DC. Characteristics and effectiveness of diabetes self-management educational programs targeted to racial/ethnic minority groups: a systematic review, meta-analysis and meta-regression. BMC Endocr Disord. 2014;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pamungkas RA, Chamroonsawasdi K, Vatanasomboon P. A Systematic Review: Family Support Integrated with Diabetes Self-Management among Uncontrolled Type II Diabetes Mellitus Patients. Behav Sci (Basel). 2017;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baig A, Benitez A, Quinn M, Burnet D. Family interventions to improve diabetes outcomes for adults. Ann N Y Acad Sci. 2015;1353:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–71. [DOI] [PubMed] [Google Scholar]
- 23.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926–43. [DOI] [PubMed] [Google Scholar]
- 24.Yeary KHK, Long CR, Bursac Z, McElfish PA. Design of a randomized controlled comparative effectiveness trial testing a Family Model of Diabetes Self-Management Education (DSME) vs. standard DSME for Marshallese in the United States. Contemp Clin Trials Commun. 2017;6:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeary KH, Aitaoto N, Sparks K, Ritok-Lakien M, Hudson JS, Goulden P, et al. Cultural Adaptation of Diabetes Self-Management Education for Marshallese Residing in the United States: Lessons Learned in Curriculum Development. Prog Community Health Partnersh. 2017;11(3):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J Statistical Power Analysis for the Behavioral Sciences 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.American Association of Diabetes Educators. AADE7 Self-Care Behaviors. Chicago, IL: American Association of Diabetes Educators; 2014. [Google Scholar]
- 28.Knowler W, Barrett-Connor E, Fowler S, Hamman R, FLachin J, Walker E, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Internal Clinical Guidelines Team. Type 2 Diabetes in Adults: Management. London: National Institute for Health and Care Excellence; 2015. 2015/12. [PubMed]
- 30.Lavelle D, Zeitoun J, Stern M, Butkiewicz E, Wegner E, Reinisch C. Diabetes self-management education in the home. Cureus. 2016;8(7):e710. [DOI] [PMC free article] [PubMed] [Google Scholar]


