Figure 1.
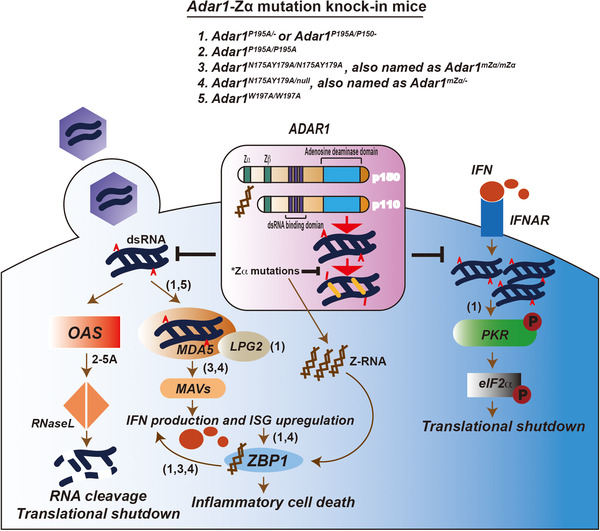
ADAR1‐Zα participates in immune responses. Upon the invasion of pathogens like viruses, dsRNAs are released into cytosol. Acting as non‐self nucleic acids, they are detected by MDA5 to induce the type I IFN signaling and by OAS to activate RNase L‐mediated widespread RNA degradation. Type I IFN binds to its receptor IFNAR which further leads to the upregulation of some endogenous dsRNAs which activate PKR and results in translational shutdown. The adenosine to inosine (A to I) editing of dsRNA by ADAR1 protects them from being sensed by MDA5, OAS or PKR, thus to block the subsequent pathways. Zα domain of ADAR1 contributes to its RNA‐editing efficiency and thus affects the inhibition of the signaling pathways indicated. Mice carrying mutations within Zα domain were characterized by their roles on the hyper‐activation of the downstream signaling pathways, separately. Specifically, P195A mutation in one Adar allele paired with the full Adar null allele (Adar1P195A/‐ ) or the Adar p150 null allele (Adar1P195A/p150– ) leads to both type I IFN pathway activation via MDA5 and LPG2, and the PKR‐mediated integrated stress response. Homozygous mutations including Adar1N175AY179A/N175AY179A and Adar1W197A/W197A in Adar render a spontaneous induction of IFN signaling. Type I IFN signaling activation results in the IFN production and upregulation of IFN‐stimulated gene (ISG), with ZBP1 is one of them. In Adar1P195A/– , Adar1P195A/p150‐ or Adar1N175AY179A/null mice, IFN activation compared with unsupervised Z‐RNA due to the loss‐of‐function of ADAR1‐Zα mutation trigger a ZBP1‐mediated inflammatory cell death, which is responsible partially for the postnasal lethality in those mice. Moreover, ZBP1 seems also play a role in augmenting IFN signaling.
