FIGURE 1.
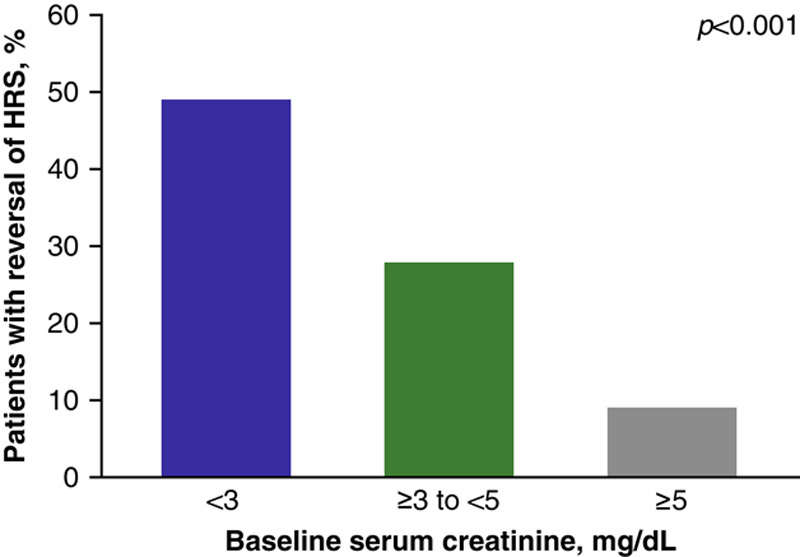
Percent of patients in the terlipressin treatment group who had HRS reversala by baseline serum creatinine subgroup (terlipressin group, pooled ITT populationb). aThe incidence of HRS reversal across the 3 Phase III studies was defined as at least 1 serum creatinine value of ≤1.5 mg/dL while on treatment (on treatment was defined as up to 24 h after the final dose of study drug) by Day 14 or discharge. †Pooled data were collated from the following Phase III studies: OT-0401, REVERSE, and CONFIRM. Abbreviations: HRS indicates hepatorenal syndrome; ITT, intent-to-treat.
