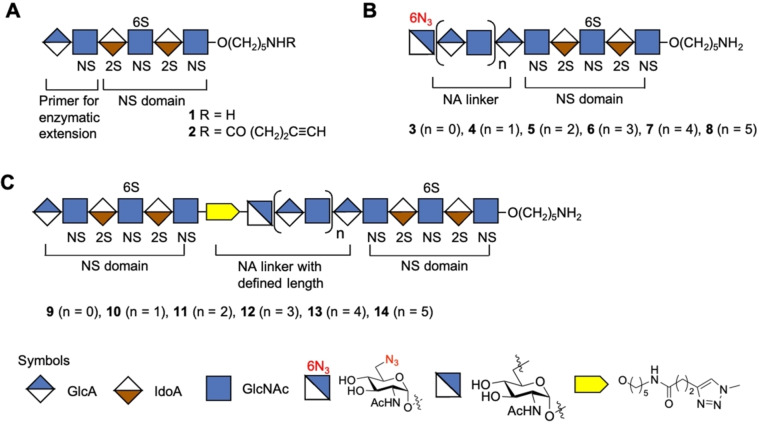
Figure 2.
Synthetic strategy for the preparation of HS mimetics having well‐defined NA and NS domains. A) Hexasaccharide 1 that terminates in a GlcA moiety, which is a primer for PmHS2 enzymatic extension. Compound 2 is equipped with an alkyne moiety at the reducing end of 1. B) Enzymatically extended compounds 3, 4, 5, 6, 7, and 8 that terminate in a GlcNAc‐6N3 moiety and repeated GlcA‐GlcNAc moiety. C) CuAAC click reaction products 9, 10, 11, 12, 13, and 14 in which NS domains are separated by an NA domain of defined length. Symbol nomenclature for HS backbone monosaccharides, structure of azide modified GlcNAc, and inter‐domain triazole linkage is presented. 2S 2‐O‐sulfate, 6S 6‐O‐sulfate and NS N‐sulfate.