Figure 3.
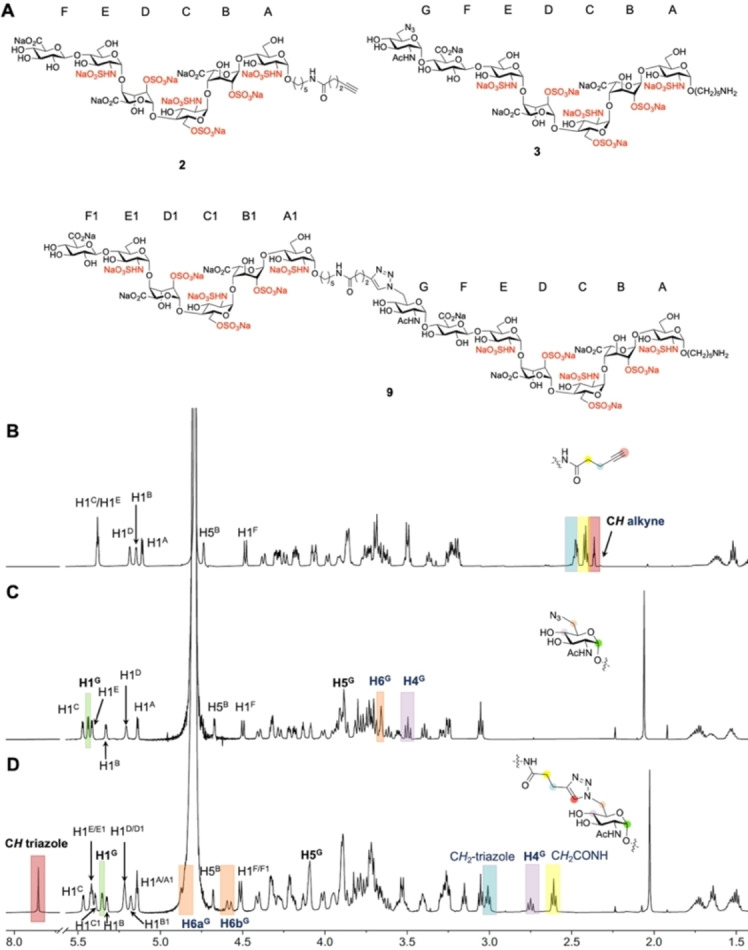
Analysis of synthetic compounds by NMR spectroscopy. 1H NMR stacked plots and structures of compounds 2, 3, and their CuAAC product 9. A) Structures of compounds 2, 3, and 9, sugar rings are labelled alphabetically, starting from reducing to non‐reducing end. B) 1H NMR of hexasaccharide 2 where anomeric linker is extended with alkyne functionality; characteristic protons are annotated. C) 1H NMR of compound 3 with terminal GlcNAc‐6N3; characteristic protons are annotated. D) 1H NMR of CuAAC product of 2 and 3, HS mimetic 9. Blue highlighted area, presence of CH2 that is attached to alkyne of compound 2 (B) and presence of CH2 that is attached to triazole of compound 9 (D). Yellow highlighted area, presence of CH2 that is highlighted in the structure (B and D). Red highlighted area, presence of CH alkyne of compound 2 (B) and presence of CH triazole of compound 9 (D). Orange highlighted area, presence of H6 of GlcNAc‐6N3 of compound 3 (C) and presence of H6 of sugar with triazole of compound 9 (D). Purple highlighted area, presence of H4 of GlcNAc‐6N3 of compound 3 (C) and presence of H4 of sugar with triazole of compound 9 (D). Green highlighted area, presence of H1 of GlcNAc‐6N3 of compound 3 (C) and presence of H1 of sugar with triazole of compound 9 (D).
