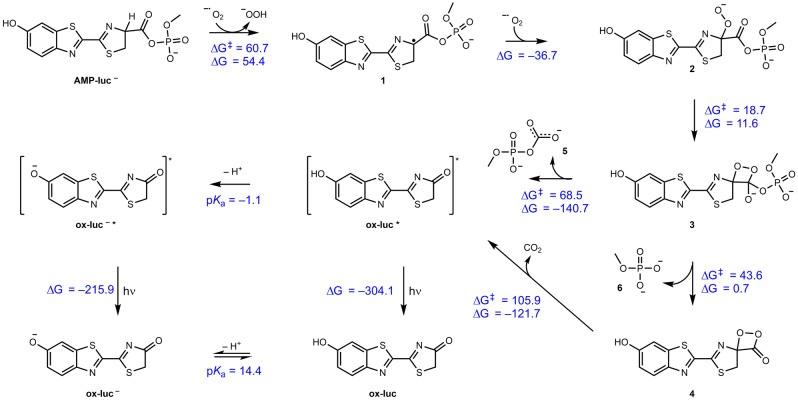
Scheme 3.
Proposed mechanism of the AMP‐luc electrochemically generated light path, supported by first principles calculated Gibbs free reaction energies and barriers (298 K, kJ mol−1) as obtained with wB97XD/Def2‐TZVP//M062X/6–31+G(d,p) using SMD to model the DMSO solvent environment. Electrochemically generated superoxide abstracts a hydrogen atom to generate a radical intermediate (1) that is kinetically trapped by a barrierless radical combination with a second superoxide molecule. This undergoes a mildly endergonic rearrangement to yield an endoperoxide intermediate (3), which is kinetically trapped by a highly exergonic oxidative decarboxylation to yield the excited state of ox‐luc (ox‐luc*), which in turn emits light upon relaxation to the product ox‐luc. Sequential loss of the phosphate to yield 4 followed by decarboxylation to yield the excited state of ox‐luc* was also considered but is less kinetically feasible. The excited state has a pK a of −1.1 and thus in principle could deprotonate if the reaction is kinetically competitive with relaxation to the ground state. In the ground state the pK a is 14.4 and the neutral form is preferred.