Figure 1.
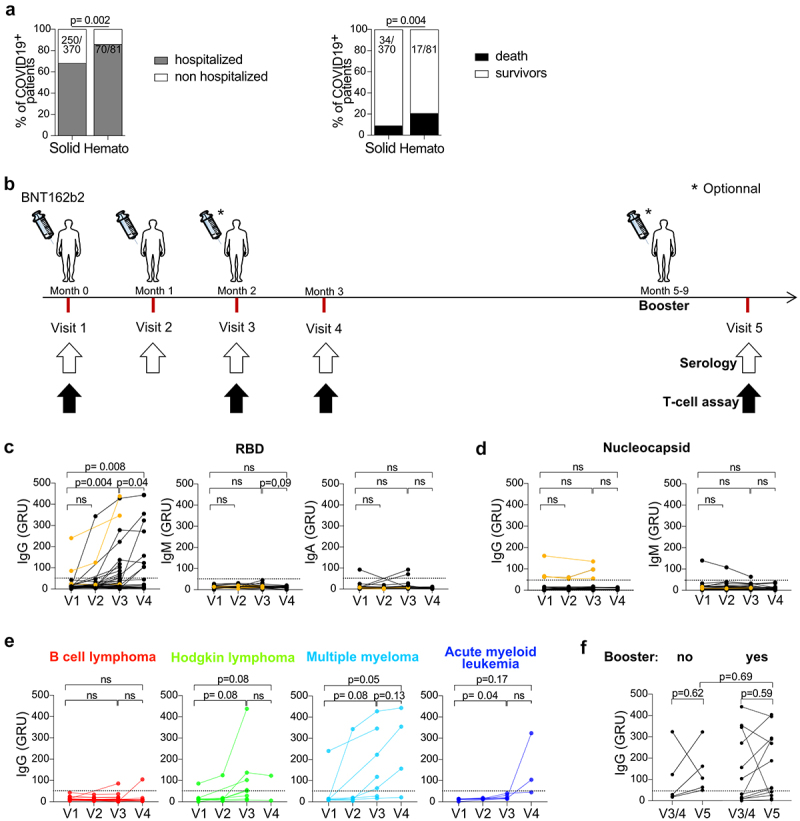
Antibody responses after BNT162b2 injections in HM patients. a. SARS-CoV-2 related hospitalization rates and mortality rates observed in Gustave Roussy Cancer Campus from March 2020 to February 2021, prior to vaccination, in patients with solid tumors and hematologic malignancies. b. Vaccination schedule and sample collection. Study participants received two or three priming doses of BNT162b2 on months 0, 1 and 2 and a booster dose at least 5 months after the last priming dose. Sera and PBMC were obtained on month 0 (pre-priming), 1, 2 and 3 and one month after the booster dose. c. Anti-CoV-2 S1-RBD IgG, IgM and IgA levels were measured in the sera during the priming dose injections. d. Anti-CoV-2 nucleocapsid (NC) IgG and IgM levels were measured in the sera. The three HM patients previously infected with SARS-CoV-2 are depicted in orange. e-f. Anti-CoV-2 S1-RBD IgG levels are shown according to HM type (e) and booster dose (f) (yes, n = 12, no, n = 5). Chi2 test analysis was performed for a. Paired student t-tests were performed for c-f.
GRU: Genalyte Reactive Unit.
