Keywords: fatigue, long COVID, physical activity, post-COVID-19 syndrome, quality of life
Abstract
The aim of this study was to determine the effectiveness of physical exercise, respiratory muscle training, and the self-management World Health Organization (WHO) recommendations leaflet on the recovery of physical fitness, quality of life, and symptom status in people with post-COVID-19 conditions. Eighty nonhospitalized adults with a post-COVID-19 condition were randomly assigned to one of four 8-wk parallel intervention groups: 1) multicomponent exercise program based on concurrent training (CT, number of subjects (n) = 20; 3 resistance and endurance supervised sessions per week at low-moderate intensity); 2) inspiratory muscle training (RM, n = 17; 2 standardized daily sessions); 3) a combination of both of the above (CTRM, n = 23); and 4) control group (CON, n = 20; following the WHO guidelines for post-COVID-19-related illness rehabilitation). No significant differences between groups were detected at baseline. Although no significant differences between interventions were detected in the V̇o2max, significant individual improvements were identified in the CT (7.5%; effect size, ES = 0.28) and CTRM (7.8%; ES = 0.36) groups. Lower body muscle strength significantly improved in the CT and CTRM (14.5%–32.6%; ES = 0.27–1.13) groups compared with RM and CON (−0.3% to 11.3%; ES = 0.10–0.19). The CT and CTRM groups improved significantly for dyspnea and fatigue, as did the health status. In addition, significant differences between interventions were described in fatigue and depression scales favoring CT and CTRM interventions. An individualized and supervised concurrent training with or without inspiratory muscle training was safe and more effective than self-care recommendations and inspiratory muscle training alone, to regain cardiovascular and muscular fitness, improve symptom severity, and health status in outpatients with post-COVID-19 conditions.
NEW & NOTEWORTHY Eight weeks of concurrent training, with or without inspiratory muscle exercise, was better than WHO “Support for Rehabilitation: Self-Management after COVID-19-Related Illness” recommendations or inspiratory muscle training alone to improve cardiopulmonary fitness, strength, and symptom severity, in a safe and effective manner. The RECOVE trial proved the benefits and utility of a supervised exercise program in people with post-COVID-19 conditions after mild COVID-19 in an ambulatory setting.
INTRODUCTION
According to the World Health Organization (WHO) consensus definition, a post-COVID-19 condition “occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 mo from the onset of COVID-19, with symptoms that last for at least 2 mo and which cannot be explained by an alternative diagnosis” (1). These post-COVID-19 conditions have also been commonly referred to as long COVID or post-COVID-19 syndrome. This affects patients who have had an acute SARS-CoV-2 infection, both severe and mild, characterized by a varied set of symptoms such as fatigue, postexertional malaise, shortness of breath (dyspnea), cognitive impairment, headache, and musculoskeletal pain, among others (2) and which more importantly, have a generally negative impact on everyday functioning (3). The erratic nature of symptoms, fluctuating in intensity and relapsing over time, and their long-term persistence, has led many of these patients to a deterioration in their quality of life (4). Although it is difficult to estimate the prevalence of persistent symptoms in people who have not required hospitalization, it is estimated to be between 3% and 12% (5, 6).
Except for vaccination, which appears to have a protective effect (7, 8), no pharmacological therapy is being used outside of clinical trials (9). In fact, guidelines on clinical management of nonhospitalized patients with post-COVID-19 conditions are usually limited to providing information, self-management leaflets, peer support, and other symptom management strategies (10). One example of this type of recommendation is the brochure produced by the WHO on “Support for Rehabilitation: Self-Management after COVID-19-Related Illness” that continues to be a reference in the outpatient setting (10, 11). Nonetheless, due to the complex nature of this syndrome, a rehabilitative intervention that includes a multidimensional program is required (12) and, exercise could be an important element in post-COVID-19 rehabilitation (12, 13). However, no specific training has been recommended, and further research to determine the effectiveness of exercise interventions for this population is needed.
We proposed that the training principles of other cardiometabolic, rheumatological, or respiratory diseases (14–16), based on concurrent training (CT) and respiratory muscle training (MR), could be adapted to post-COVID-19 conditions. These chronic noncommunicable diseases are characterized by chronic persistent low-grade inflammation and endothelial damage, as has been described in long COVID (17). Exercise, in addition to regaining physical function, could relieve symptom burden, improve health-related quality of life and, through its anti-inflammatory properties and its enhancing effect on the immune system, improve patients after COVID-19 infection (18, 19). Therefore, a tailored, supervised concurrent training program, could offer a nonpharmacological alternative to improve post-COVID-19 conditions.
The RECOVE trial (REhabilitation for post-COVID-19 condition through a supervised Exercise intervention) evaluated nonhospitalized people with post-COVID-19 conditions, to identify the role of an exercise program, based on multicomponent exercise training and/or inspiratory muscle training, compared with the WHO self-management recommendations leaflet commonly used in outpatient scenarios, on the recovery of persistent symptoms and functional limitations after COVID-19. We hypothesized that the supervised exercise programs would be better in improving physical and mental status after 8 wk of physical training compared with the conventional recommendations.
METHODS
Study Population
This study was registered at ClinicalTrials.gov (NCT04718506), approved by an ethical review board by Murcia University Ethics Committee (Reference No. 3447/2021), and reported according to the CONSORT (Consolidated Standards of Reporting Trials) statement. Written informed consent was obtained from all study participants.
Participants were recruited through advertisements on social media or via general practitioners. Inclusion criteria were subjects aged over 18 yr who had a confirmed microbiological diagnosis of COVID-19 by SARS-CoV-2 reverse transcription-polymerase chain reaction on an oropharyngeal-nasopharyngeal swab or a positive rapid antigen test, who presented a chronic symptomatic phase, lasting >12 wk from the onset of symptoms, and had not been hospitalized because of the acute COVID-19 infection. Those with evidence of COVID-19 pneumonia needed a Brixia score ≤ 5 (20) and to show total recovery of pulmonary function and radiological follow-up. None must have received specific SARS-CoV-2 treatment. We excluded pregnant women and those who had acute or unstable chronic diseases such as unstable myocardiopathy, ischemic heart disease, uncontrolled hypertension, asthma, or chronic obstructive pulmonary disease (COPD), and those who had major surgery in the past 3 mo.
Study Design
The RECOVE trial is an 8-wk, four-arm, parallel experimental design. After baseline measurements, a V̇o2max-stratified computer-generated randomization sequence, with 1:1:1:1 allocation to either one of the 8-wk programs, was created.
Participants were assigned to a supervised concurrent training program [with inspiratory muscle training (CTRM) or without it (CT)], to an autonomous inspiratory muscle training program (RM), or to the control group (CON), informed to follow the WHO guideline: “Support for Rehabilitation: Self-Management after COVID-19-Related Illness” (11).
Interventions
Concurrent training.
Participants from the CT and CTRM groups completed a tailored multicomponent exercise program adapted from the ACSM (American College of Sports Medicine) guidelines for chronic obstructive pulmonary disease and cardiovascular disease (21). Familiarization sessions were run during the first week of the concurrent training program. They completed a three-days-a-week concurrent training routine: two days of resistance training [50% 1RM (one-repetition maximum), 3 sets, 8 repetitions, 4 exercises (squat, bench press, deadlift, and bench pull)] followed by moderate intensity variable training [MIVT: 4–6 × 3–5 min at 70%–80% heart rate reserve (HRR)/2–3 min at 55%–65% HRR], and one day of a monitored autonomous light intensity continuous training (LICT: 30–60 min, 65%–70% HRR). A weekly linear programming model (volume was varied in session or set of sessions) was conducted for endurance sessions.
In addition to monitoring heart rate (HR) and individualizing strength training loads by movement velocity control in each session (22), the subjective rate of perceived exertion (RPE) was continuously assessed with a visual scale in all sessions (RPE, according to the Borg scale: 6–20). A target score between 11 and 12 in LICT and strength training and without exceeding a score of 16 in MIVT was used. This RPE monitoring (23, 24) allowed us to control the exertion intensity of patients with difficulties in reaching the estimated heart rate due to severe dyspnea, serious fatigue, or chronotropic incompetency (25).
All sessions were directed by certified strength and conditioning coaches and conducted under medical supervision. Attendance ≥ 85% (at least 20 of the 24 scheduled sessions) was mandatory to continue in the study.
Inspiratory muscle training.
Participants in CTRM and RM groups performed an inspiratory muscle training protocol with PowerBreath Classic Heath Series mechanic threshold devices. All subjects performed 1 set of 30 repetitions [62.5 ± 4.6% of the PIM (maximum inspiratory pressure)], preceded by a warm-up set, twice a day, every day of the week (26, 27).
Participants were trained by the medical team on the setup and use of the resistance device in a familiarization session before the first session. They were instructed that the resistance should be increased every two weeks by turning the load adjustment clockwise ¼ to 1 full turn, pending on tolerance to maintain a 12–15 RPE on Borg scale 6–20. They were also provided with written instructions on the technical characteristics of the device, possible adverse events, and the manufacturer’s recommendations.
Adherence was controlled through online forms that each participant had to fill out weekly. A minimum of 10 weekly sessions was considered necessary to form part of the study.
Nonsupervised self-management recommendations.
Participants from the control group (CON) were advised to follow the WHO guidelines: “Support for Rehabilitation: Self-Management after COVID-19-Related Illness” (11), as a home-based program. They were informed about the recommendations and the leaflet was sent by email. As in real-life practice, indications were not directly supervised nor adherence monitored ensuring that spontaneous compliance to treatment was not modified.
The WHO exercise recommendations included a gradually increasing activity program using five stages controlled by the Borg scale of perceived exertion (RPE: 0–10): 1) Phase 1: Preparing to return to exercise (RPE: 0–1): controlled breathing exercises, light walk and stretching, and balance exercises, 2) Phase 2: Low-intensity activity (RPE: 2–3): walking, light housework/yard work for 15 min a day, for 1 wk, 3) Phase 3: Moderate-intensity activity (RPE: 4–5): brisk walking, going up and down stairs, jogging for up to 30 min, and introducing resistance exercises (biceps curls, wall pushes, arm raises, sit to stand, knee straightening, squats, and heel raises) for 15 min. Participants were required to continue in this phase for at least one week, 4) Phase 4: Moderate-intensity exercises with coordination and running skills (RPE: 5–7), running, cycling, swimming, and dance classes. If RPE score for any of these exercises was more than 7, the participant should return to the previous phase, and 5) Phase 5: Return to baseline exercise (RPE: 8–10): Participant was able to complete their usual pre-COVID-19 exercise/sports/activity regimen until the 8th wk of follow-up.
Measurements
Baseline characteristics and anthropometric measures.
Detailed baseline data including demographics, past medical history, medications, toxic habits, vital signs, and physical examination were obtained by an infectious disease’s consultant in a face-to-face preparticipatory interview. Spirometry, screening ECG (electrocardiogram), and echocardiography were evaluated before cicloergometer effort test by a cardiology team before randomization. Any cardiac SARS-CoV-2 infection long-term complication or unnoticed cardiac comorbidity were excluded.
Body composition (height, body mass, % of fat mass, and lean body mass) and body mass index (BMI) were measured by a multifrequency segmental body bioelectrical impedance analyzer (Tanita MC-780U, Tokyo, Japan).
Main outcomes.
Cardiorespiratory fitness.
Participants completed a submaximal multistage and individualized cardiopulmonary exercise test on a cycle ergometer (Ergoline, Ergoselect 200) according to the Ekblom-Bak protocol (28). The test is suitable in situations when a maximal test is not feasible, for example, in health evaluations. Mean heart rate during the last minute at the higher work rate was recorded. Outcome of exercise test was the estimated V̇o2max (mL/kg/min). The sex-based equations to calculate V̇o2max have a strong correlation between the estimated and the actual value, with an r2 = 0.86 for men and r2 = 0.83 for women, with a coefficient of variation (CV) 8.7% and Standard Error of the Estimate (SEE) 0.28 L·min−1 (29).
Muscle strength.
The individual load-velocity relationships in the bench press (BP) and half squat (HSQ) exercises were determined by means of a progressive loading test up to the 1RM (30, 31). Following a standardized warm-up, the initial load was set at 5 kg and was gradually increased in 5 kg increments until the attained mean propulsive velocity (MPV) was ≤0.60 m·s−1 for HSQ and ≤0.80 m·s−1 for BP. The heaviest load that each participant could properly lift using a full range of motion and without external help, was considered his 1RM. All lifts during testing and training were conducted using a Smith machine (Multipower Fitness Line, Peroga, Murcia, Spain) with no counterweight mechanism. Repetitions evaluated during testing and training sessions were recorded by using a linear velocity transducer with a sampling frequency of 1,000 Hz (T-Force System, Ergotech, Murcia, Spain). A very high test-retest reliability of this testing protocol (intraclass correlation coefficients, ICC = 0.99, 95% CI = 0.99–1.00; Coefficient of Variation, CV = 2.5%) has been recently described (32). Outcomes from the following neuromuscular parameters were considered for the analysis: 1RM strength and average MPV attained against common absolute loads to PRE and POST assessments (MPVALL).
Maximal isometric hand grip strength was determined on the dominant hand using a digital dynamometer (TKK5101, Grip-D, Takey, Tokyo, Japan). The average result of two repetitions was recorded (kp). The assessment was conducted with the subject standing, shoulders neutrally rotated and adducted, and forearm in neutral.
Secondary outcomes.
Severity of symptoms.
Patient-reported outcomes measures (PROM) included health-related quality of life by the 12-item Short Form Survey (SF-12), calculating the mental component (MH) and physical activity (PA) domain scores (33). Anxiety and depression symptoms were calculated using the General Anxiety Disorder Questionnaire-7 (GAD-7) (34) and the Patient Health Questionnaire-9 (PHQ-9) (35). A cut-off score for moderate-severe depression and anxiety ≥ 10 points was considered for secondary analyses. Perception of dyspnea was estimated using the Modified Medical Research Council Dyspnea Scale (mMRC) (36). A cut-off score for severe breathlessness ≥ 2 was considered for secondary analyses. Fatigue intensity was determined using the average score on Fatigue Severity Scale (FSS) (37) and Chalder Fatigue Scale (CFS) using the Linkert scoring system (38). Scores of ≥4 in FSS and ≥18 in CFS indicate severe fatigue. Functional limitations after COVID-19 were calculated using the Post-COVID-19 Functional Status (PCFS) scale (39).
Statistical Analysis
Standard statistical methods were used for the calculation of mean, standard deviation (SD), median, interquartile range (IQR), and standard error of the means (SEM). Assumption of normality was verified using the Shapiro–Wilk test and the homogeneity of variance across groups (CT, RM, CTRM, and CON) using the Levene’s test. A 2 (group) × 2 (time: pre vs. post) factorial analysis of variance with Bonferroni adjustment was used to analyze the differences between groups. One-way ANOVA was also run to compare the percentage of change scores (Δ%) between pre versus post time points in the main outcomes. McNemar’s test was applied to identify differences in categorical PROMs. Effect size (ES) was estimated by Cohen’s d for factorial analyses (0.3 small, 0.5 moderate, and 0.7 large) and Cohen’s g for McNemar’s analyses (0.05 small, 0.15 medium, and 0.25 large). Statistical significance was established at the P < 0.05 level. Analyses were performed using SPSS software version 25.0 (IBM Corp., Armonk, NY) and the rcompanion and Cohen G packages for RStudio (v.2021.09.1). Determination of efficacy was based on the per-protocol (PP) population, which consisted of all participants who were randomized and completed the assigned programs.
RESULTS
Eighty patients were included in the per-protocol analysis, per group: CT (n = 21), CTMR (n = 25), RM (n = 17), and CON (n = 20). Three patients dropped out of the study for reasons unrelated to symptom worsening: one due to moderate SARS-CoV-2 reinfection (CTRM group), one due to instability of psychiatric pathology and adherence problems (CTRM group), and one due to lack of commitment to the program (CT). No adverse event occurred during the training sessions. The mean number of supervised training sessions performed by participants in the concurrent training groups was 88.0% (CT = 87.3% and CTRM = 88.7%, with no differences between groups). Furthermore, 100% of patients in the RM group reported follow-up information. The median number of declared session compliance to RM training was 12 of 14 per week.
Baseline Characteristics
The main basic characteristics of the sample as well as the evolution of symptom characteristics are shown in Table 1. All patients except one (Arab) were Caucasian. There were no significant differences between groups in any of the baseline characteristics, nor for any of the symptoms referred (P = 0.270–0.983). As the patients were stratified by main outcome (estimated V̇o2max), there were no significant differences between groups (P = 0.372).
Table 1.
Main baseline characteristics of the sample and pre-post intervention frequency of symptoms evolution
| Total (n = 80) | CON (n = 20) | CT (n = 20) | CTRM (n = 23) | RM (n = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 45.3 ± 8.0 | 47.8 ± 7.6 | 44.3 ± 9.6 | 44.1 ± 7.4 | 43.0 ± 6.9 | |||||
| Female sex | 55 (69) | 14 (70) | 14 (70) | 17 (74) | 10 (59) | |||||
| BMI, kg/m2 | 26.6 ± 4.6 | 26.0 ± 3.7 | 25.7 ± 3.9 | 26.7 ± 5.2 | 28.0 ± 5.5 | |||||
| Weeks of symptoms | 39.3 ± 23.3 | 39.4 ± 24.7 | 40.8 ± 24.5 | 43.0 ± 24.8 | 49.0 ± 22.5 | |||||
| Comorbidity | ||||||||||
| Nonpsychiatric* | 19 (24) | 3 (15) | 4 (20) | 6 (26) | 6 (35) | |||||
| Mood disorders | 34 (42) | 5 (25) | 8 (40) | 13 (56) | 8 (47) | |||||
| Medication | ||||||||||
| Taking any medication | 55 (69) | 13 (65) | 14 (70) | 17 (73) | 11(64) | |||||
| Antidepressants | 23 (29) | 8 (40) | 7 (35) | 5 (21) | 3 (17) | |||||
| Benzodiazepines | 25 (31) | 7 (35) | 7 (35) | 7 (30) | 4 (23) | |||||
| Bronchodilators | 16 (20) | 5 (25) | 3 (15) | 3 (13) | 5 (30) | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of symptoms† | 8 ± 3 | 6 ± 4# | 8 ± 4 | 7 ± 5# | 7 ± 3 | 4 ± 3# | 9 ± 3 | 6 ± 4# | 8 ± 3 | 7 ± 5# |
| Moderate or severe | 56 (70) | 37 (46)# | 15 (75) | 10 (50) | 10 (50) | 5 (25) | 18 (78) | 12 (52) | 13 (76) | 10 (59) |
| Symptoms (order of frequency) | ||||||||||
| Fatigue† | 69 (86) | 46 (57)# | 16 (80) | 15 (75) | 17 (85) | 7 (35)# | 21 (91) | 14 (61)# | 15 (88) | 10 (59) |
| Dyspnea† | 55 (69) | 35 (44)# | 13 (65) | 14 (70) | 12 (60) | 2 (10)# | 17 (74) | 11 (48)# | 13 (76) | 8 (47) |
| Lack of concentration | 51 (64) | 40 (50)# | 12 (60) | 11 (55) | 13 (65) | 6 (30)# | 15 (65) | 12 (52) | 11 (65) | 11 (68) |
| Memory problems | 51 (64) | 46 (57) | 12 (60) | 10 (50) | 12 (60) | 9 (45) | 16 (70) | 16 (70) | 11 (65) | 11 (65) |
| Brain fog | 49 (61) | 39 (49)# | 11 (55) | 9 (45) | 11 (55) | 6 (30) | 18 (78) | 13 (56) | 9 (53) | 11 (65) |
| Sleep disturbances | 47 (59) | 31 (39)# | 11 (55) | 6 (30) | 9 (45) | 5 (26) | 14 (61) | 12 (52) | 13 (76) | 8 847) |
| Myalgia | 45 (56) | 33 (42) | 11 (55) | 9 (45) | 9 (45) | 5 (25) | 15 (65) | 13 (59) | 10 (59) | 6 (35) |
| Low mood | 42 (52) | 25 (31)# | 11 (55) | 7 (35) | 8 (40) | 3 (15)# | 13 (57) | 8 (34)# | 10 (59) | 7 (41) |
| Headache | 41 (51) | 32 (40)# | 10 (50) | 6 (30) | 8 (40) | 6 (30) | 14 (61) | 10 (45) | 9 (53) | 9 (53) |
| Anxiety† | 36 (45) | 30 (37) | 9 (45) | 5 (25) | 7 (35) | 4 (20) | 14 (61) | 11 (48) | 6 (35) | 10 (59) |
Data are frequencies and percentages [n (%)] or means and standard deviation (M ± SD). BMI, body mass index; CON, control group; CT, concurrent training group; CTRM, concurrent training + respiratory muscle training group; ES, effect size; RM, respiratory muscle training group.
Nonpsychiatric comorbidity includes asthma [n = 10, (12.5%)], hypertension [n = 6 (7.5%)], diabetes [n = 3 (3.8%)], structural heart disease [n = 3, (3.8)], cerebrovascular disease [n = 1 (1.3%)], and COPD (chronic obstructive pulmonary disease) [n = 1, (1.3%)]. There were no significant differences between groups in any of the baseline characteristics. †Favors CT and CTRM interventions, P < 0.005; #pre-post significant differences (P < 0.05). n represents number of subjects.
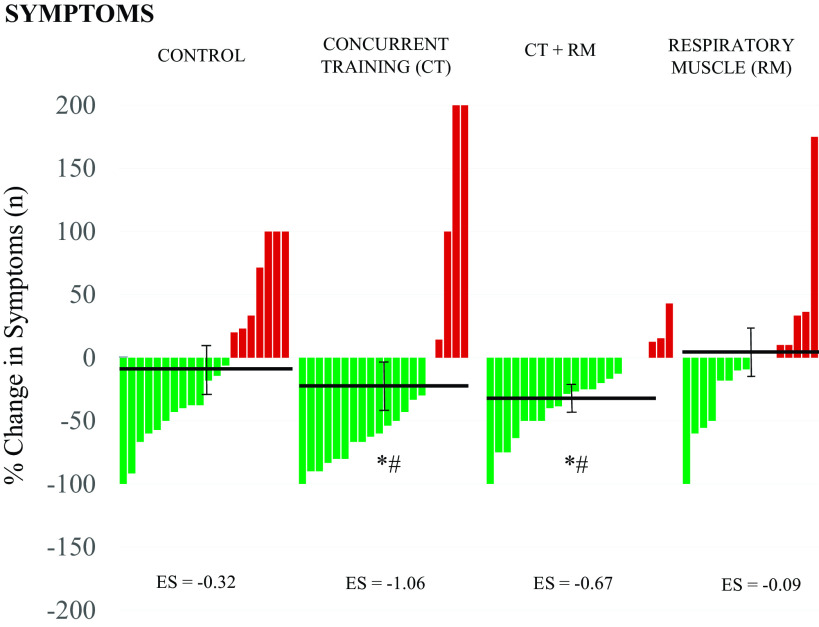
After 8 wk of follow-up, the total number of symptoms decreased significantly in the full sample, as did the percentage of patients who considered their symptoms to be moderate or severe (P < 0.001). The symptoms decreased on average: −3 for CT and CTMR and −1 for RM and CON groups. The percentage of change of each participant according to intervention group is shown in Fig. 1. The concurrent training groups (CT and CTRM) had a significant reduction in symptoms with respect to the RM and CON groups (P < 0.05). Dyspnea, fatigue, and anxiety have also significantly improved in favor of CT and CTRM groups (Δ −50 to 13%, P < 0.05). No differences in any other symptom prevalence, including headache, brain fog, or neurocognitive manifestations, were found between interventions (Table 1).
Figure 1.
Changes in total number of symptoms after 8 wk of follow-up. Data expressed with means ± SEM and effect size (ES). *Statistically significant differences from CON group, #statistically significant differences from RM group. CON, control.
Main Outcomes
Following the 8 wk-intervention period, no significant differences between groups were detected in the estimated V̇o2max (P > 0.05), even though significant individual improvements were identified in the CT (Δ 7.5%; P < 0.05) and CTRM (Δ 7.8%; P < 0.05) groups (Fig. 2A).
Figure 2.
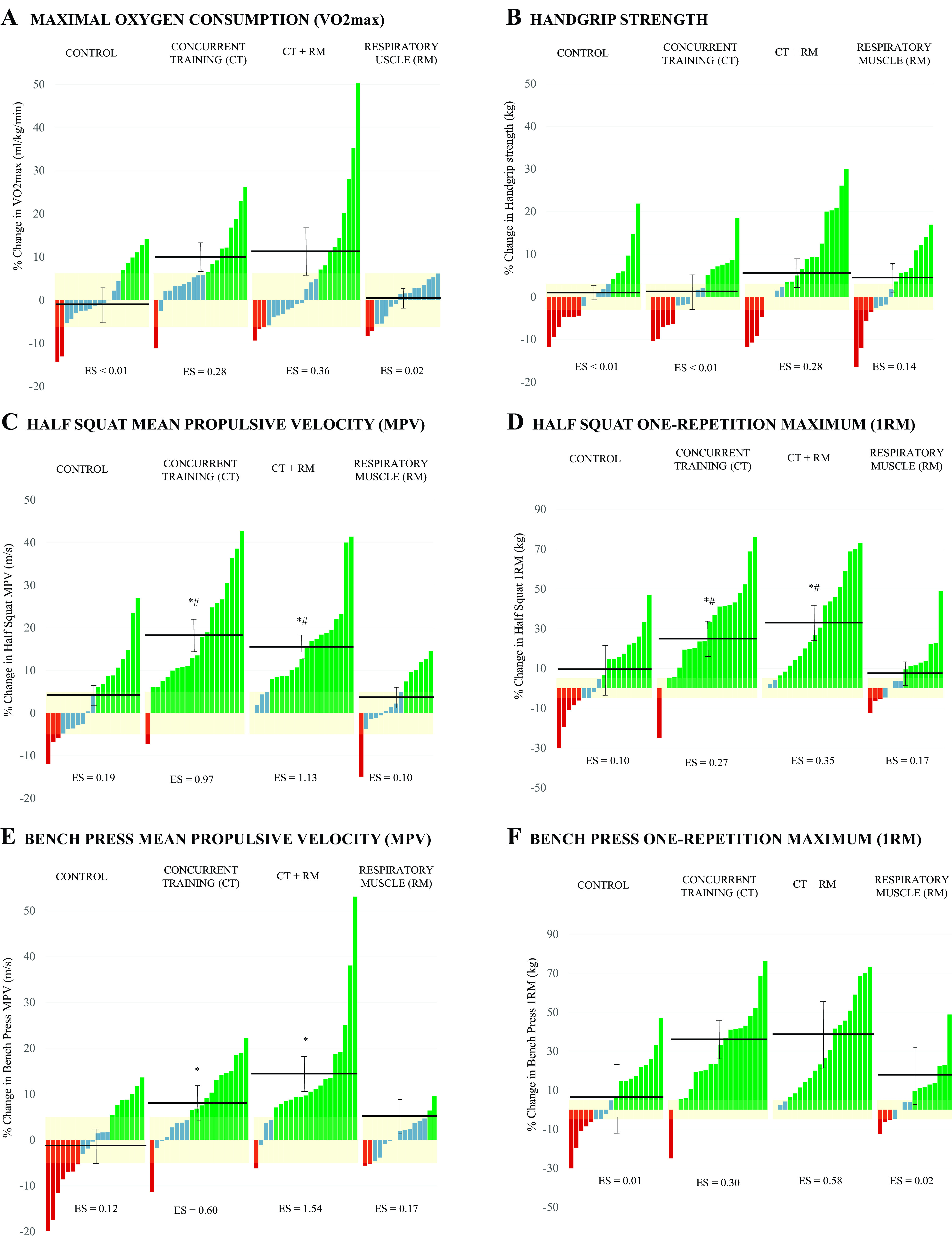
Changes in selected cardiorespiratory and muscle strength variables from pre to post intervention for each group. Each bar represents a subject. Groups’ means and SEM of the change are indicated by the horizontal and error lines. A: V̇o2max. B: hand grip strength. C: half squat mean propulsive velocity (MPV). D: half squat one repetition maximum (1RM). E: bench press mean propulsive velocity (MPV). F: bench press one repetition maximum (1RM). *Statistically significant differences from CON group. #Statistically significant differences from RM group. Bar colors mean positive responders (green), nonresponders (blue), and negative responders (red), considering the measurement error of the tests (yellow shadow). CON, control; ES, effect size.
Lower body maximal and submaximal strength (squat 1RM and MPVALL) significantly improved for both groups who accomplished the multicomponent exercise training (Δ 14.5–32.6%; P < 0.05), whereas no changes were detected for RM or CON groups (Δ −0.3 to 11.3%; P > 0.05; Fig. 2, C and D). In addition, significant interaction was found for upper body submaximal strength (Bench Press MPVALL) (P < 0.05) for CT and CTRM groups, whereas significant pre-post improvements were detected in maximal (1RM) and submaximal strength (MPVALL) for BP following these interventions (Δ 7.8–39.5%; P < 0.05), without relevant changes in the MR and CON groups (Δ −1.4 to 3.8%; P > 0.05; Fig. 2, E and F). No inter- or intragroup interactions were found for the dominant hand grip strength (P > 0.05) (Fig. 2B).
The individual changes experienced in each group for the main outcomes are also represented in Fig. 2. In the concurrent training groups, the percentage of responders who increased the estimated V̇o2max was between 30% and 40%, and over 80% in strength outcomes (1RM and MPVALL), reaching 95% in submaximal strength in HSQ.
Secondary Outcomes (PROM)
At the end of follow-up, the entire cohort significantly improved scores on all PROMs. The PCFS scale decreased from a median of 3 (IQR 2–3) to 1 (IQR 1–3), (P < 0.001). Similarly, the number of participants with mMRC < 2 increased from 55% to 79% (P < 0.001). Participants with a score ≥10 on depression and anxiety scale significantly decreases from 65% to 35% and from 50% to 27.5% respectively (both, P < 0.001). The same thing happened on the FSS < 4 (18.8%–42.5%, P < 0.001) and CSF-Linkert <18 (28.7%–57.5%, P < 0.001). SF-12 PA and mental health (MH) domains also showed relevant differences (SF-12 PA 35.2–42.9; P < 0.001 and SF-12 MH, 41.4–44.6; P = 0.002).
Pre-post intervention differences in PROMs are listed in Table 2. After 8 wk-intervention period, no significant differences between groups were detected in the mMRC (dyspnea), GAD-7 (anxiety), PCFS (functional status), and SF-12 PA and MH (health-related quality of life). Fatigue (FFS and CFS-Linkert) and depression (PHQ-9) significantly improved in concurrent training groups (P = 0.007–0.039). Pre-post measurements for participants in the CT and CTRM groups improved significantly in all PROMs [with the exceptions of the SF-12 MH (P = 0.09) and GAD-7 < 10 and mMRC in the CT group (both P = 0.063) and the PCFS < 2 in the CTRM (P = 0.102)]. Changes were also observed in SF-12 PA domain for RM group. No relevant change was observed in the control group in pre-post analysis for any of these variables.
Table 2.
Pre-post intervention follow-up data for PROM
| CON |
CT |
CTRM |
RM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | |
| SF-PA | 36.5 ± 11.7 | 38.5 ± 13.4 | - | 35.2 ± 11.6 | 48.2 ± 10.4** | 1.18 | 33.8 ± 10.3 | 41.0 ± 10.8** | 0.60 | 35.8 ± 10.4 | 44.1 ± 10.4* | 0.64 |
| SF-MH | 39.5 ± 12.1 | 42.2 ± 11.6 | - | 46.3 ± 11.9 | 49.6 ± 9.5 | - | 39.5 ± 11.5 | 44.8 ± 10.9* | 0.51 | 40.3 ± 11.3 | 41.3 ± 11.5 | - |
| mMRC < 2 | 11 (55) | 13 (65) | - | 12 (60) | 17 (85) | - | 11 (48) | 19 (83)* | 0.50 | 10 (59) | 14 (82) | - |
| FSS < 4† | 4 (20) | 6 (30) | - | 6 (30) | 14 (70)** | 0.50 | 2 (9) | 8 (35)* | 0.50 | 3 (18) | 6 (35) | - |
| CFS < 18† | 4 (20) | 7 (35) | - | 5 (25) | 17 (85)** | 0.50 | 8 (35) | 15 (65)* | 0.39 | 6 (35) | 7 (41) | - |
| PHQ9 < 10† | 5 (25) | 9 (45) | - | 6 (30) | 18 (90)** | 0.50 | 10 (43) | 16 (70)* | 0.50 | 7 (41) | 9 (53) | - |
| GAD7 < 10 | 9 (45) | 12 (60) | - | 13 (65) | 18 (90) | - | 9 (39) | 17 (74)* | 0.50 | 9 (53) | 11 (65) | - |
| PCFS < 2 | 4 (20) | 9 (45) | - | 3 (15) | 14 (70)** | 0.50 | 7 (17) | 8 (35) | - | 3 (18) | 8 (47) | - |
Data are frequencies and percentages [n (%)] or means and standard deviation (M ± SD). CFS, Chalder Fatigue Scale; CON, control group; CT, concurrent training group; CTRM, concurrent training + respiratory muscle training group; ES, effect size; FSS, Fatigue Severity Scale; GAD-7, Generalized Anxiety Disorder-7; mMRC, Modified Medical Research Council Dyspnea Scale; PCFS, Post-COVID-19 Functional Status; PHQ-9, Patient Health Questionnaire-9; PROM, Patient Reported Outcome Measures; RM, respiratory muscle training group; SF-12 MH, 12-item Short Form Survey Mental Health domain; SF-12 PA, 12-item Short Form Survey Physical Activity domain.
†Favors CT and CTRM interventions, P < 0.05; Pre-post significant differences *P < 0.05, **P < 0.001.
DISCUSSION
To our knowledge, this is the first randomized clinical trial to evaluate the effect of a concurrent exercise program with or without inspiratory muscle training on physical condition and symptom perception in a cohort composed exclusively of patients with post-COVID-19 conditions after nonhospitalized mild SARS-CoV-2 infection. The results support the hypothesis that a supervised multicomponent training program confers benefits on cardiovascular fitness and muscle strength, as well as on the recovery of the physical and mental health status of these patients. The concurrent training, regardless of the addition of inspiratory muscle training, was more effective in improving the primary outcomes (estimated V̇o2max and maximal and submaximal muscle strength) as well as the two main symptoms (fatigue and dyspnea) than respiratory muscle training alone or the issuance of general nonindividualized exercise guidelines (i.e., WHO recommendations) when these are not subject to supervision or monitoring, as in real life conditions. Regarding the safety of the interventions, the benefits were achieved in the absence of complications and in the outpatient setting, with minimal medical resources and under the supervision of qualified strength and conditioning trainers, so they can be easily generalized to the population with the same selection criteria as this study.
Overall, the clinical course was satisfactory over time for the entire sample (n = 80), with or without training, both for the number of symptoms and their perceived intensity (see Table 1 and Fig. 1). This physical and mental health recovery in post-COVID-19 condition patients has recently been suggested in the literature (40). However, current research suggests that this post-COVID-19 symptomatology improvement could be significant only in the case of physical rehabilitation (CT and CTRM groups), showing that exercise plays a critical role in the acceleration of recovery compared with usual care (self-management exercise recommendations and general tips for handling symptoms). This could translate into a better ability to cope with daily life, given the relationship between the number of symptoms and their intensity, as has been previously described (41).
Remarkably, over 80% of the participants responded positively to both of the supervised training interventions (CT and CTRM, Fig. 2), demonstrating that the individual-paced approach was highly effective for most individuals. And although the commitment in the RM group was high enough (12 weekly sessions), arguably, the worst individual response in unsupervised interventions may be explained due to the lack of control in the intensity progression. We considered that a targeted supervision, for example, through the use of accelerometers, adherence questionnaires, exercise diaries, messaging reminders, etc. in the CON group would have interfered with the behavior of the participants in real life. Monitoring raises patients’ awareness of their exercise habits and it contributes to better improvements (42). Nonetheless, the use of self-care recommendations and symptomatic management of patients with post-COVID-19 conditions continues to be one of the reference treatments in primary care (10, 12). This is partly due to insufficient evidence to support exercise programs in populations with post-COVID-19 conditions that have not required hospitalization. Recent systematic reviews with meta-analyses on the role that pulmonary rehabilitation programs play in the recovery of patients with long COVID-19 (43, 44) reinforce this idea: until the RECOVE study, there had been no studies in outpatient population to support concurrent training in the ambulatory settings.
Several researchers have evaluated patients with post-COVID-19 conditions with persistent postexertional fatigue and dyspnea, finding impairments in lung function, breathing pattern, and abnormal muscular oxygen extraction when compared with healthy controls (45, 46). These factors potentially contributed to reduced maximal oxygen consumption (V̇o2max), to greater minute ventilation, and to ventilatory inefficiency (greater equivalents for carbon dioxide, V̇e/V̇co2max) during cardiopulmonary exercise test (47). In addition, a depressed chronotropic response (25) has also been described as a potential mechanism for both clinical manifestations. It has been suggested that autonomic, endothelial, and mitochondrial dysfunction are possible underlying mechanisms for these findings and could be caused by direct SARS-CoV-2 cell damage, chronic inflammation, or anomalous autoimmune responses(17). No published study has included comprehensive autonomic testing, endothelial examination, or tested direct mitochondrial function (47, 48) to prove the mechanisms by which exercise in post-COVID-19 conditions may be helpful.
The concurrent training program, with and without inspiratory muscle training, allowed the participants to recover an average V̇o2max of 2.5 mL·kg·min−1 and 2.9 mL·kg·min−1, respectively [half of the expected mean loss in patients with long COVID-19 (−4.9 mL·kg·min−1) (95% CI, −3.4 to 6.4) (47)] and significantly improve maximal and submaximal strength, with gains ranging from 20% to 40%, in just 8 wk of supervised training (Fig. 2). Strength training enables superior adaptations when performed in conjunction with resistance training in healthy and chronically ill individuals, increasing specific gains in muscle performance, resistance to fatigue, and contributing to gains in V̇o2max (49). The effect of inspiratory muscle training on estimated V̇o2max was null, supporting the fact that this type of exercise improves respiratory muscle strength but does not improve V̇o2max in healthy individuals (50). These gains in physical performance may explain the symptomatic improvement in dyspnea and fatigue in CT and CTRM groups, since V̇o2max is the main determinant of physical condition, even though no significant differences were detected between the interventions (Fig. 2A).
In addition to recovery in physical condition, concurrent training provides greater benefits on health-related quality of life and symptom intensity (Table 2). Only in the multicomponent training groups (CT and CTRM) and the RM group, were there significant improvements in health-related quality of life, dyspnea (mMRC), and fatigue (both FSS and CFS-Linkert) over time. In addition, the concurrent training strategy (CT and CTRM), alone or with respiratory muscle training, was shown to be superior in reducing fatigue and depressive symptoms than respiratory training alone or self-managed programs, as recommended by the WHO guideline. These findings are consistent with previous experiences published in the hospital setting or specialized rehabilitation centers in patients with COVID-19 pneumonia who underwent multicomponent cardiopulmonary rehabilitation (51, 52) and those in whom only respiratory muscle training was used (53). Unlike these programs, which are difficult to access for outpatients, the main advantage of our proposal is that it was designed for mild patients who currently represent the largest number of COVID-19 cases, with a low investment of resources, safely in any training center, and supervised by qualified strength and conditioning coaches.
Limitations
This work poses several limitations. First, the sample size and the characteristics of the participants, it does not allow the results to be generalized in a population that required hospitalization, other races, or child and elderly populations. Second, the lack of follow-up after its completion does not allow us to assume the sustainability of the results. Third, low levels of supervision of exercise programs in RM and CON groups, or uncontrolled intensity, may result in nonsignificant health benefits. In addition, the prognosis of patients with COVID-19 has changed over the time of the study, thanks to new treatments and vaccines, and it is not clearly known whether the vaccines have an inducing or relieving effect on the symptoms once the syndrome has developed (54). Finally, other aspects such as nutrition and hydration status, sleep and rest time, medications, and other behavioral factors, were not controlled and could have modified the findings.
Perspective and Significance
Even though there have been significant advances in the treatment, prognosis, and prevention of acute SARS-CoV-2 infection, the number of patients affected by persistent conditions due to COVID-19 continues to grow, and the science community has not been able to offer a useful strategy for these patients’ symptomatic relief. Dependence on pharmacotherapy or vaccines as the only mitigation strategies may not optimally reduce the long-term social, economic, and health consequences of SARS-CoV-2 infections.
In this scenario, a multicomponent exercise program was shown to be a safe and useful tool for improving cardiovascular fitness, muscular strength, and to ameliorate symptom burden and improve mood and quality of life in patients with post-COVID-19 conditions with low costs and minimal structural and human resources. Self-care and informed recommendations used in an ambulatory setting are highly inefficient and are not an effective treatment alternative when used in isolation. Inspiratory muscle training requires strict control and high adherence to achieve effective results. Further research exploring other combinations of training (e.g., high intensity interval training) or a combination of treatments (physical and neuropsychobiological approach) and healthy life-style interventions (nutrition, toxic habits, sleep quality) is warranted to achieve even better results.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by The University of Murcia and was partially funded by the Spanish Ministry of Science and Innovation Grant No. PID2019-108202RA-I00 and by the Centro Médico Virgen de la Caridad Agreement No. 35110.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.-A., J.C.-I., and J.G.P. conceived and designed research; A.J.-A., Á.B.-R., A.M.-C., F.F.-L., and J.C.-I. performed experiments; A.J.-A. and J.G.P. analyzed data; A.J.-A. and J.G.P. interpreted results of experiments; J.G.P. prepared figures; A.J.-A. and J.G.P. drafted manuscript; A.J.-A., J.C.-I., and J.G.P. edited and revised manuscript; A.J.-A., Á.B.-R., A.M.-C., F.F.-L., B.S.-A., J.C.-I., and J.G.P. approved final version of manuscript.
REFERENCES
- 1.World Health Organization (WHO). A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021 (Online). https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [2022 Jul 19].
- 2. Crook H, Raza S, Nowell J, Young M, Edison P. Long Covid—mechanisms, risk factors, and management. BMJ 374: n1648, 2021. [Erratum in BMJ 374: n1944, 2021]. doi: 10.1136/BMJ.N1648. [DOI] [PubMed] [Google Scholar]
- 3. Meys R, Delbressine JM, Goërtz YMJ, Vaes AW, Machado FVC, Van Herck M, Burtin C, Posthuma R, Spaetgens B, Franssen FME, Spies Y, Vijlbrief H, Van’T Hul AJ, Janssen DJA, Spruit MA, Houben-Wilke S. Generic and respiratory-specific quality of life in non-hospitalized patients with COVID-19. J Clin Med 9: 3993, 2020. doi: 10.3390/JCM9123993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans N, Hereth B, Tubig P, Sorrels A, Muldoon A, Hills K, Evans NG. Long covid and disability: a brave new world. BMJ 378: e069868, 2022. doi: 10.1136/BMJ-2021-069868. [DOI] [PubMed] [Google Scholar]
- 5. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 400: 452–461, 2022. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Office for National Statistics. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK (Online). https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/4august2022 [2022 Aug 6].
- 7. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 28: 1461–1467, 2022. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 377: e069676, 2022. doi: 10.1136/BMJ-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CADTH (Canada’s Drug and Health Technology Agency). Post–COVID-19 Condition Scoping Review (Online). https://www.cadth.ca/post-covid-19-condition-scoping-review [2022 Aug 7].
- 10. Greenhalgh T, Sivan M, Delaney B, Evans R, Milne R. Long covid—an update for primary care. BMJ 378: e072117, 2022. doi: 10.1136/BMJ-2022-072117. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Support for Rehabilitation: Self-Management After COVID-19-Related Illness, Second Edition (Online).https://www.who.int/europe/publications/i/item/WHO-EURO-2021-855-40590-59892 [2022 Oct 11].
- 12.CDC (Centers for Disease Control and Prevention). Post-COVID Conditions: Information for Healthcare Providers (Online). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html#management [2022 Oct 31].
- 13.National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 (Online).https://app.magicapp.org/#/guideline/EQpzKn/section/nYe9Pn [2022 Oct 11]. [PubMed]
- 14. Moreno-Cabañas A, Ortega JF, Morales-Palomo F, Ramirez-Jimenez M, Alvarez-Jimenez L, Mora-Rodriguez R. Concurrent endurance and resistance training enhances muscular adaptations in individuals with metabolic syndrome. Scand J Med Sci Sports 31: 1440–1449, 2021. doi: 10.1111/SMS.13950. [DOI] [PubMed] [Google Scholar]
- 15. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol 11: 86–97, 2015. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 16. Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J 12: 2178–2188, 2018. doi: 10.1111/CRJ.12905. [DOI] [PubMed] [Google Scholar]
- 17. Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong C, Cleemput I, Van den Heede K. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 54: 1473–1487, 2022. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nieman DC. Exercise is medicine for immune function: implication for COVID-19. Curr Sports Med Rep 20: 395–401, 2021. doi: 10.1249/JSR.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 19. Ezzatvar Y, Ramirez-Velez R, Izquierdo M, Garcia-Hermoso A. Physical activity and risk of infection, severity and mortality of COVID-19: a systematic review and non-linear dose-response meta-analysis of data from 1 853 610 adults. Br J Sports Med 56: 1188–1193, 2022. doi: 10.1136/bjsports-2022-105733. [DOI] [PubMed] [Google Scholar]
- 20. Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med 125: 509–513, 2020. doi: 10.1007/S11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riebe D, Ehrman JK, Liguori G, Magal M. ACSM’s Guidelines for Exercise Testing and Prescription (10th ed.). Philadelphia, PA: Wolters Kluwer, 2018. [Google Scholar]
- 22. Hernández-Belmonte A, Martínez-Cava A, Morán-Navarro R, Courel-Ibáñez J, Pallarés JG. A comprehensive analysis of the velocity-based method in the shoulder press exercise: stability of the load-velocity relationship and sticking region parameters. Biol Sport 38: 235–243, 2021. doi: 10.5114/BIOLSPORT.2020.98453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 113: 147–155, 2013. doi: 10.1007/S00421-012-2421-X. [DOI] [PubMed] [Google Scholar]
- 24. Hernández-Belmonte A, Courel-Ibáñez J, Conesa-Ros E, Martínez-Cava A, Pallarés JG. Level of effort: a reliable and practical alternative to the velocity-based approach for monitoring resistance training. J Strength Cond Res 36: 2992–2999, 2022. doi: 10.1519/JSC.0000000000004060. [DOI] [PubMed] [Google Scholar]
- 25. Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Courel-Ibáñez J. Chronotropic incompetence in non-hospitalized patients with post-COVID-19 syndrome. J Clin Med 10: 5434, 2021. doi: 10.3390/jcm10225434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pick HJ, Faghy MA, Creswell G, Ashton D, Bolton CE, McKeever T, Lim WS, Bewick T. The feasibility and tolerability of using inspiratory muscle training with adults discharged from the hospital with community-acquired pneumonia. Adv Respir Med 89: 216–220, 2021. doi: 10.5603/ARM.A2021.0002. [DOI] [PubMed] [Google Scholar]
- 27. Mcconnell K, Griffiths LA. Acute cardiorespiratory responses to inspiratory pressure threshold loading. Med Sci Sports Exerc 42: 1696–1703, 2010. doi: 10.1249/MSS.0b013e3181d435cf. [DOI] [PubMed] [Google Scholar]
- 28. Ekblom-Bak E, Björkman F, Hellenius ML, Ekblom B. A new submaximal cycle ergometer test for prediction of VO2max. Scand J Med Sci Sports 24: 319–326, 2014. doi: 10.1111/SMS.12014. [DOI] [PubMed] [Google Scholar]
- 29. Björkman F, Ekblom-Bak E, Ekblom Ö, Ekblom B. Validity of the revised Ekblom Bak cycle ergometer test in adults. Eur J Appl Physiol 116: 1627–1638, 2016. doi: 10.1007/S00421-016-3412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martínez-Cava A, Morán-Navarro R, Hernández-Belmonte A, Courel-Ibáñez J, Conesa-Ros E, González-Badillo JJ, Pallarés JG. Range of motion and sticking region effects on the bench press load-velocity relationship. J Sports Sci Med 18: 645–652, 2019. [PMC free article] [PubMed] [Google Scholar]
- 31. Martínez-Cava A, Morán-Navarro R, Sánchez-Medina L, González-Badillo JJ, Pallarés JG. Velocity- and power-load relationships in the half, parallel and full back squat. J Sports Sci 37: 1088–1096, 2019. doi: 10.1080/02640414.2018.1544187. [DOI] [PubMed] [Google Scholar]
- 32. Courel-Ibáñez J, Martínez-Cava A, Morán-Navarro R, Escribano-Peñas P, Chavarren-Cabrero J, González-Badillo JJ, Pallarés JG. Reproducibility and repeatability of five different technologies for bar velocity measurement in resistance training. Ann Biomed Eng 47: 1523–1538, 2019. doi: 10.1007/S10439-019-02265-6. [DOI] [PubMed] [Google Scholar]
- 33. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International quality of life assessment. J Clin Epidemiol 51: 1171–1178, 1998. doi: 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 34. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166: 1092–1097, 2006. doi: 10.1001/ARCHINTE.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16: 606–613, 2001. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 93: 580–586, 1988. doi: 10.1378/CHEST.93.3.580. [DOI] [PubMed] [Google Scholar]
- 37. Krupp LB, Larocca NG, Muir Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46: 1121–1123, 1989. doi: 10.1001/ARCHNEUR.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 38. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. J Psychosom Res 37: 147–153, 1993. doi: 10.1016/0022-3999(93)90081-P. [DOI] [PubMed] [Google Scholar]
- 39. Klok FA, Boon GJAM, Barco S, Endres M, Miranda Geelhoed JJ, Knauss S, Rezek SA, Spruit MA, Vehreschild J, Siegerink B. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J 56: 2001494, 2020. doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, Liu M, Wang Y, Cui D, Wang Y, Zhang X, Shang L, Zhong J, Wang X, Wang J, Cao B. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 10: 863–876, 2022. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jimeno-Almazán A, Martínez-Cava A, Buendía-Romero Á, Franco-López F, Sánchez-Agar JA, Sánchez-Alcaraz BJ, Tufano JJ, Pallarés JG, Courel-Ibáñez J. Relationship between the severity of persistent symptoms, physical fitness, and cardiopulmonary function in post-COVID-19 condition. A population-based analysis. Intern Emerg Med 7: 2199–2208, 2022. doi: 10.1007/S11739-022-03039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larsen RT, Wagner V, Korfitsen CB, Keller C, Juhl CB, Langberg H, Christensen J. Effectiveness of physical activity monitors in adults: systematic review and meta-analysis. BMJ 376, 2022. doi: 10.1136/BMJ-2021-068047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soril LJJ, Damant RW, Lam GY, Smith MP, Weatherald J, Bourbeau J, Hernandez P, Stickland MK. The effectiveness of pulmonary rehabilitation for post-COVID symptoms: a rapid review of the literature. Respir Med 195: 106782, 2022. doi: 10.1016/j.rmed.2022.106782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fugazzaro S, Contri A, Esseroukh O, Kaleci S, Croci S, Massari M, Facciolongo NC, Besutti G, Iori M, Salvarani C, Costi S. Rehabilitation interventions for post-acute COVID-19 syndrome: a systematic review. Int J Environ Res Public Health 19: 5185, 2022. doi: 10.3390/ijerph19095185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, Parati G. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985) 130: 1470–1478, 2021. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frésard I, Genecand L, Altarelli M, Gex G, Vremaroiu P, Vremaroiu-Coman A, Lawi D, Bridevaux PO. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. BMJ Open Respir Res 9: e001126, 2022. doi: 10.1136/BMJRESP-2021-001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Durstenfeld MS, Sun K, Tahir P, Peluso MJ, Deeks SG, Aras MA, Grandis DJ, Long CS, Beatty A, Hsue PY. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open 5: e2236057, 2022. doi: 10.1001/jamanetworkopen.2022.36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Boer E, Petrache I, Goldstein NM, Olin JT, Keith RC, Modena B, Mohning MP, Yunt ZX, San-Millán I, Swigris JJ. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am J Respir Crit Care Med 205: 126–129, 2022. doi: 10.1164/rccm.202108-1903LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cadore EL, Pinto RS, Lhullier FLR, Correa CS, Alberton CL, Pinto SS, Almeida APV, Tartaruga MP, Silva EM, Kruel LFM. Physiological effects of concurrent training in elderly men. Int J Sports Med 31: 689–697, 2010. doi: 10.1055/S-0030-1261895. [DOI] [PubMed] [Google Scholar]
- 50. Fernández-Lázaro D, Gallego-Gallego D, Corchete LA, Fernández Zoppino D, González-Bernal JJ, García Gómez B, Mielgo-Ayuso J. Inspiratory muscle training program using the PowerBreath®: does it have ergogenic potential for respiratory and/or athletic performance? A systematic review with meta-analysis. Int J Environ Res Public Health 18: 6703, 2021. doi: 10.3390/IJERPH18136703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barbara C, Clavario P, De Marzo V, Lotti R, Guglielmi G, Porcile A, Russo C, Griffo R, Mäkikallio T, Hautala AJ, Porto I. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur J Prev Cardiol 29: e258–e260, 2022. doi: 10.1093/eurjpc/zwac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, Koczulla AR, Ay C, Zwick RH. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration 101: 593–601, 2022. doi: 10.1159/000522118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McNarry MA, Berg RMG, Shelley J, Hudson J, Saynor ZL, Duckers J, Lewis K, Davies GA, Mackintosh KA. Inspiratory muscle training enhances recovery post COVID-19: a randomised controlled trial. Eur Respir J 2103101, 2022. doi: 10.1183/13993003.03101-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strain WD, Sherwood O, Banerjee A, Van der Togt V, Hishmeh L, Rossman J. The impact of COVID vaccination on symptoms of long COVID: an international survey of people with lived experience of long COVID. Vaccines 10: 652, 2022. doi: 10.3390/VACCINES10050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.