Table 5. CD19 positive B-cell and CD20 positive B-cell counts at measured time points.
| Variables | Counts (pcs/µL) (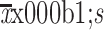 ) [reduction (%)]* ) [reduction (%)]* |
P** | |
| Ripertamab (N=42) | Rituximab# (N=42) | ||
| pcs, pieces. *, reduction (%) was calculated based on the mean values of the counts at baseline and the counts at the time points post infusion of study drugs; **, t-test or Wilcoxon rank-sum test was used for the inter-group comparison; #, rituximab refers to MabThera®. | |||
| CD19-positive B-cells | |||
| Baseline | 154.67±137.16 | 132.51±125.77 | 0.448 |
| D 1 post infusion | 4.19±8.69 (97.3) | 3.98±5.58 (97.0) | 0.488 |
| D 2 post infusion | 3.91±6.15 (97.5) | 2.78±3.25 (97.9) | 0.454 |
| D 7 post infusion | 2.38±4.21 (98.5) | 1.42±2.18 (98.9) | 0.430 |
| D 29 post infusion | 2.01±3.58 (98.7) | 2.28±3.55 (98.3) | 0.344 |
| D 57 post infusion | 1.84±4.39 (98.8) | 2.42±5.08 (98.2) | 0.337 |
| D 85 post infusion | 4.12±9.05 (97.3) | 4.78±11.67 (96.4) | 0.450 |
| CD20-positive B-cells | |||
| Baseline | 157.78±138.42 | 134.70±127.01 | 0.434 |
| D 1 post infusion | 0.77±1.49 (99.5) | 1.21±4.00 (99.1) | 0.419 |
| D 2 post infusion | 0.72±1.44 (99.5) | 0.80±2.13 (99.4) | 0.395 |
| D 7 post infusion | 0.77±1.94 (99.5) | 0.54±1.36 (99.6) | 0.348 |
| D 29 post infusion | 0.71±1.57 (99.6) | 0.71±1.43 (99.5) | 0.310 |
| D 57 post infusion | 1.70±4.98 (98.9) | 1.47±5.59 (98.9) | 0.324 |
| D 85 post infusion | 3.37±9.88 (97.9) | 3.08±7.87 (97.7) | 0.374 |
