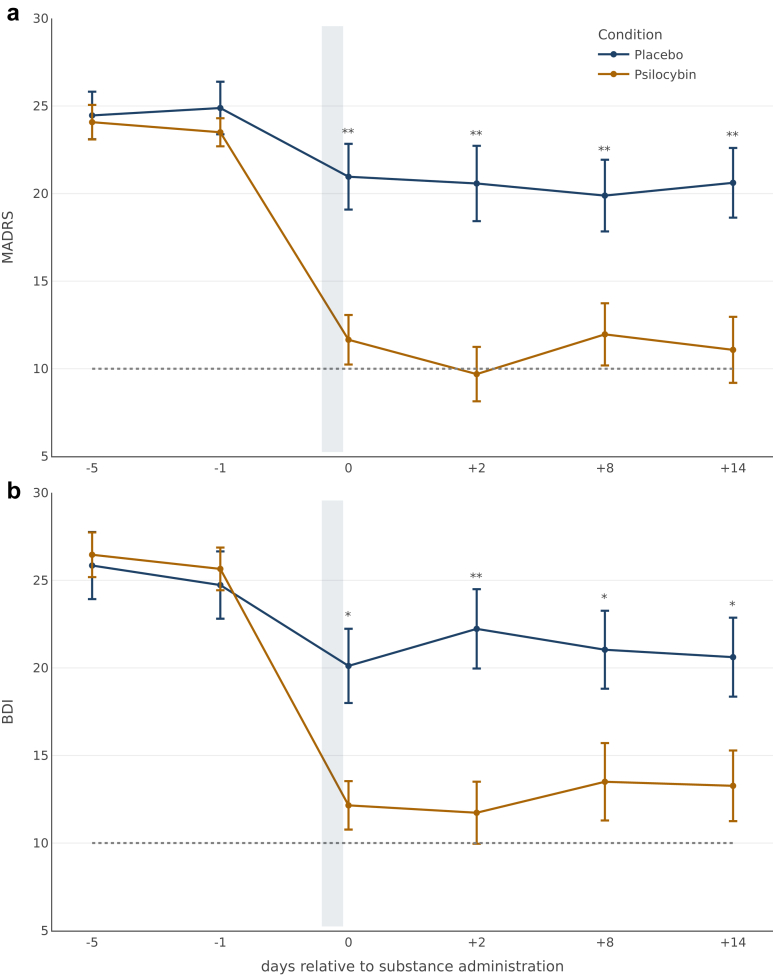
Fig. 2.
Mean trajectories of MADRS and BDI at every study visit for both treatment conditions. a: mean scores of clinician-rated primary endpoint (MADRS); b: mean scores of self-reported primary endpoint (BDI). The grey bar depicts the time of substance administration. The endpoints reported for the administration day were assessed after all subjective effects had worn off (around 6 h after administration). The dotted line depicts thresholds defined for the remission of symptoms. Differences between treatment conditions (blue = psilocybin; yellow = placebo) at each time point were calculated using independent two-samples Welch's t-tests rejecting the null-hypothesis at significance levels of ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. Error bars represent standard errors of means (se).