Figure 6.
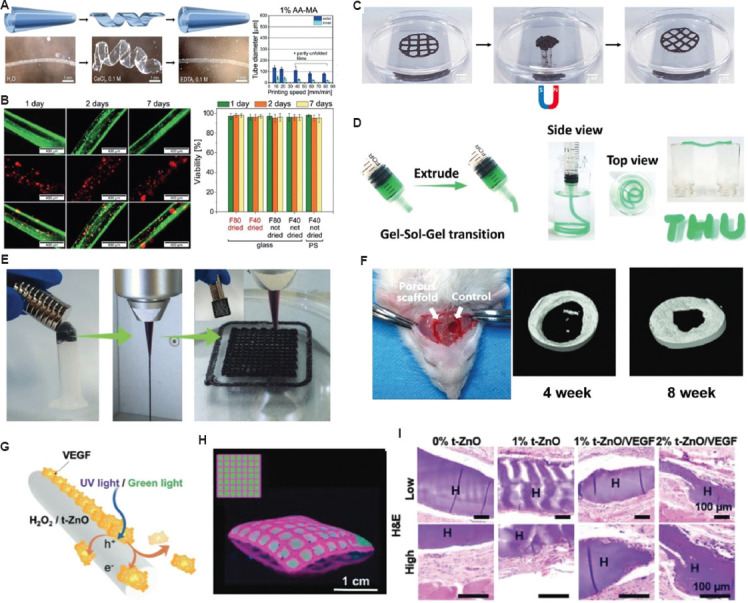
Potential applications of stimuli-responsive bioinks for tissue regeneration and repair. (A) AA-MA tube responsiveness in immersed solutions and tube diameters according to printing speed. (B) Representative fluorescent images of cell-laden AA-MA tubes for 7 days: fluorescence images in green (upper row) representing live cells, fluorescence images in red (middle row) showing dead cells within self-folded tubes, and overlays of green and red fluorescence images (lower row) with live cells (orange) and dead cells (red)[152]. (Figure A and B reproduced with permission from Kirillova et al.[152]; copyright 2017, John Wiley and Sons). (C) Alteration in the fabricated 3D structure by applying a magnetic field; the originally printed shape was returned when the magnetic field was removed (reproduced with permission from Ko et al.[113]; copyright 2020, Elsevier). (D) Images showing the injected bioink through a syringe to create self-supporting structure. (E) The images of magnetic ferrofluids, extrusion filament, and 3D-fabricated structure with magnetic-responsive behavior. (F) Micro-CT images after implantation on 4 weeks and 8 weeks. (Figure D, E, and F reproduced with permission from Guo et al.[114]; copyright 2021, John Wiley and Sons). (G) Working principle showing drug-release through UV/green light stimulations. (H) Micropatterned patch by 3D bioprinting process. (I) Hematoxylin and eosin staining of skin tissue collected after 28 days to show the wound healing process. (Figures G, H, and I reproduced with permission from Siebert et al.[116]; copyright 2021, John Wiley and Sons).
