FIGURE 4.
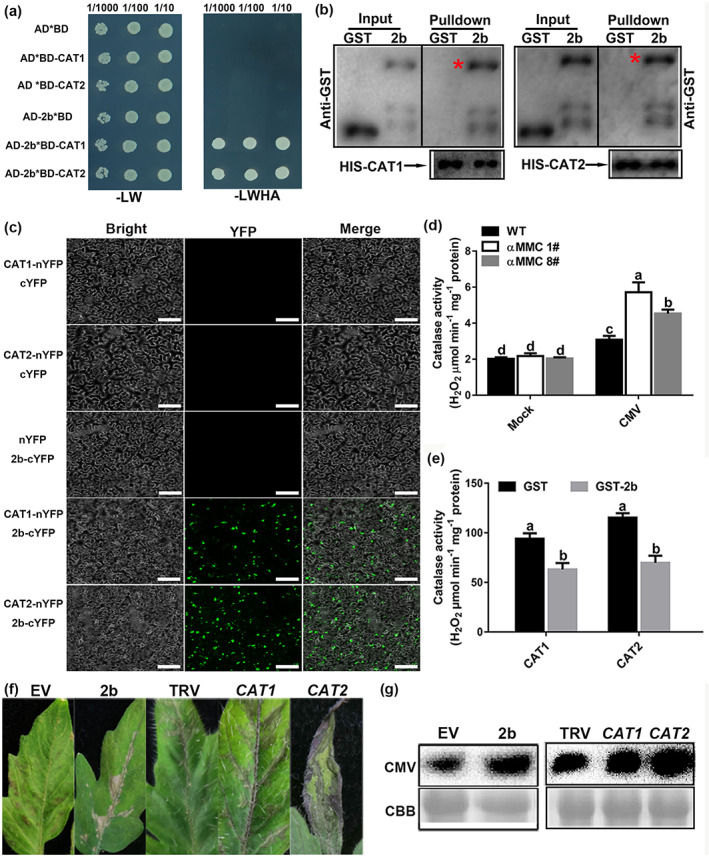
CMV 2b directly interacts with catalases in vivo and inhibits their activities to promote CMV accumulation. (a) CMV 2b interacts with catalases in yeast. Yeast growth on QDO medium lacking Leu, Trp, His, and Ade (−LWHA) illustrated the interaction. (b) Pull‐down assays examining the interactions among 2b, CAT1, and CAT2 in vitro. Purified 2b‐GST or glutathione‐S‐transferase (GST) was incubated with CAT1‐His and CAT2‐His. After immunoprecipitation with His beads, the proteins were monitored by anti‐CAT or anti‐GST antibodies. Red asterisks indicate bands of the target protein. (c) Bimolecular fluorescence complementation (BiFC) analyses of the interactions between catalases and 2b in Nicotiana benthamiana leaves. Green fluorescence illustrates the interaction, bars = 40 μm. (d) Measured catalase activity in wild‐type (WT) and αMMC transgenic plants in response to CMV infection. (e) Detection of CAT1 and CAT2 enzymatic activity in reactions containing 2b‐GST or GST. CAT was incubated with 2b at 37°C. (f) Disease symptoms of CMV infection in the empty vector (EV), 2b‐overexpressing, nonsilenced control (TRV alone), and CAT1‐ or CAT2‐silenced tomato leaves by TRV‐induced gene silencing. (g) Detection of CMV coat protein accumulation in EV, 2b‐overexpressing, TRV‐silenced, CAT1‐silenced, and CAT2‐silenced tomato by western blotting. Systemic leaves were harvested for analysis. Coomassie brilliant blue (CBB) staining of RuBisCO protein indicates the loading control. Values indicate means ± SD from three independent experiments and lowercase letters represent significant differences (p < 0.05)
