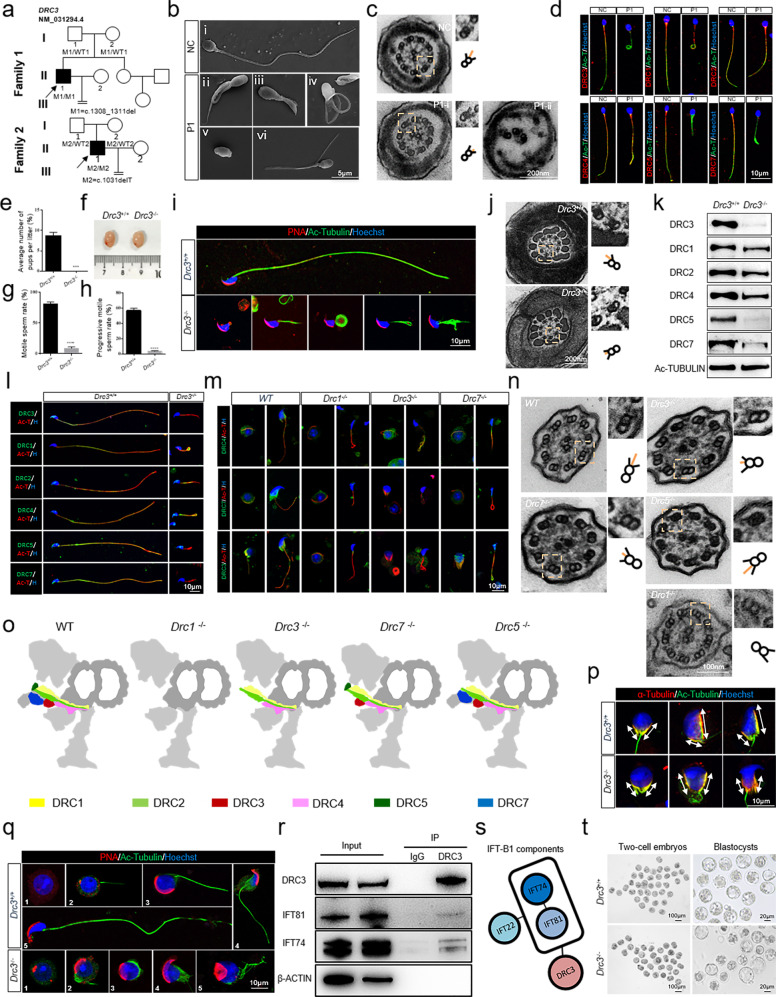
Fig. 1.
DRC3 functions as an assembly adapter for nexin–dynein regulatory complex (N-DRC) functional components during spermatogenesis in humans and mice. a Pedigrees of DRC3 variants in two men with asthenoteratozoospermia. Filled and open signs indicate the affected and unaffected individuals, respectively. Arrows indicate the probands. Double horizontal lines indicate a consanguineous marriage. P1: patient1, M1: c.1308_1311del, P2: patient2, M2: c.1031delT (NCBI accession no.: NM_031294.4). b Scanning electron microscopy analysis of the spermatozoa obtained from a normal control (NC) and P1. Spermatozoa from the NC showed normal long flagella (i), whereas spermatozoa from P1 showed coiled flagella (ii), short flagella (iii), irregular flagella (iv), absent flagella (v), and angulation flagella (vi). Scale bar = 5 μm. c Transmission electron microscopy (TEM) analysis of the cross-section of spermatozoan flagella from the NC and P1. The dashed boxes mark a set of microtubular doublets and the attached accessories, including the inner and outer dynein arms and the N-DRC. Structurally complete N-DRCs were found in spermatozoan flagella from the NC, whereas the complete “9 + 2” structure with a truncated N-DRC (P1-i) (20.6%) and disordered microtubules (P1-ii) (79.4%) were detected in P1 samples. d Immunofluorescence analysis showed that DRC1, DRC2, and DRC4 could be detected, whereas DRC3, DRC5, and DRC7 were almost undetectable in the spermatozoa of obtained from P1 (red: DRCs, green: Ac-tubulin, blue: Hoechst). Scale bar = 10 μm. e An average of nine pups per litter was observed for Drc3+/+ mice, whereas Drc3−/− male mice failed to sire any pups. (*** indicates P < 0.001). f The testis sizes were comparable between Drc3+/+ and Drc3−/− mice at 8 weeks of age; n = 3. (g–h) The spermatozoa of Drc3−/− mice showed dramatic decreases in motility and progressive motile ability compared to those from Drc3+/+ mice (****P < 0.0001); n = 3. i Spermatozoa from Drc3+/+ mice showed integral, long flagella, whereas spermatozoa from Drc3−/− mice displayed multiple flagellum abnormalities. j TEM analysis indicated that the N-DRC structure of the sperm flagellum axoneme from Drc3−/− mice was obviously shorter than that from Drc3+/+ mice. The dashed boxes mark a set of microtubular doublets and the attached accessories, including the inner and outer dynein arms, and N-DRC; scale bar = 200 nm. k–l In sperm from Drc3−/− mice, DRC3, DRC5, and DRC7 were almost undetectable, whereas DRC1, DRC2, and DRC4 levels were comparable with those in sperm from Drc3+/+ mice based on western blot analysis (k) and by fluorescence analysis (red: DRCs, green: Ac-tubulin, blue: Hoechst); scale bar = 10 μm (l). Ac-tubulin was used as the control. m In the spermatids from the testicular suspension, DRC4 could be detected in wild-type (WT), Drc3 and Drc7-knockout mice, but was absent in Drc1−/− mice (upper), respectively. DRC7 was absent in Drc1−/−, Drc3−/− and Drc7−/− mice (middle). DRC3 was absent in Drc1−/− and Drc3−/− mice, but remained in Drc7-/-mice (lower) (red: Ac-tubulin; green: DRC4, DRC7 and DRC3; blue; Hoechst. Scale bar = 10 μm). n The observation of N-DRC structures in of tracheal cilia via TEM indicated the normal and long N-DRCs in WT and Drc5-null mice, shorter N-DRCs in Drc3−/− and Drc7−/− mice, and the loss of N-DRCs in Drc1−/− mice; scale bar = 100 nm. o Pattern diagrams of the N-DRC complex in WT and in Drc1−/−, Drc3−/−, Drc7−/−, and Drc5−/−mice. p The manchettes of Drc3+/+ mice displayed asymmetric contraction, whereas those manchettes of Drc3−/− mice showed a symmetrical change. The two-way arrow indicates the manchette in the spermatids; scale bar = 10 μm. q Spermatids from a testicular suspension of Drc3+/+ mice (above) showed an extended axoneme (1–4) and an integral, long tail (5), whereas spermatids of Drc3-/- mice (below) showed an abnormal flagellum axoneme extension (1–4) and deformed tail (5). r The results of immunoprecipitation in vivo revealed that DRC3 might interact with IFT81 and IFT74. s Pattern diagram of the interaction between DRC3 and the detected IFT-B1 components. The circle represents the DRC3 and intraflagellar transport (IFT) proteins, and the line segment represents the interactive relationship. t Representative two-cell embryos and blastocysts from Drc3+/+ and Drc3−/− groups, indicating that impaired fertilization capability caused by Drc3 dysfunction can be rescued via intracytoplasmic sperm injection (ICSI) treatment