Abstract
Hypertension is the main cause of cardiovascular disease, especially in women. Black women (58%) are affected by higher rates of hypertension than other racial/ethnic groups contributing to increased cardio‐metabolic disorders. To decrease blood pressure (BP) in this population, a pilot randomized controlled trial was conducted to examine the effects of Interactive Technology Enhanced Coaching (ITEC) versus Interactive Technology (IT) alone in achieving BP control, adherence to antihypertensive medication, and adherence to lifestyle modifications among Black women diagnosed with and receiving medication for their hypertension. Participants completed a 6‐week Chronic Disease Self‐Management Program (CDSMP), and 83 participants were randomly assigned to ITEC versus IT. Participants were trained to use three wireless tools and five apps that were synchronized to smartphones to monitor BP, weight, physical activity (steps), diet (caloric and sodium intake), and medication adherence. Fitbit Plus, a cloud‐based collaborative care platform was used to collect, track, and store data. Using a mixed‐effects repeated measures model, the main effect of group means indicated no significant difference between the treatment and referent groups on study variables. The main effect of time indicated significant differences between repeated measures for systolic BP (p < .0001), weight (p < .0001), and steps (p = .018). An interaction effect revealed differences over time and was significant for study measures except diastolic BP. An important goal of this preliminary analysis is to help Black women prioritize self‐care management in their everyday environment. Future research is warranted in a geographically broader population of hypertensive Black women.
Keywords: Black women, coaching, hypertension, interactive technology, lifestyle modification
1. INTRODUCTION
Hypertension, currently defined as a systolic blood pressure (SBP) ≥130 mmHg or diastolic (DBP) ≥80 mmHg, 1 , 2 is a public health burden globally 3 and in the United States. 2 Worldwide, hypertension is the main risk factor for cardiovascular disease in women contributing to premature death. 4 Almost half of all adults 20 years of age and older (47%) in the United States have hypertension, with a lower prevalence among White (41%), Hispanic (41%), and Asian (42%) women. However, Black women (58%) are affected by higher hypertension‐related diseases and cardio‐metabolic disorders. 2 , 5
The high prevalence of hypertension in Black adults is poorly understood because of the multifactorial and synergistic effect of psychosocial, socioeconomic, behavioral, and environmental factors that augment stress and influence access to care and health outcomes. 6 Regardless of the socioeconomic status of Black people, the effects of physiological and mental stress associated with racism and discrimination are ever‐present and contribute to the development of hypertension and other chronic diseases. 7 As multiple social issues further the complexity of hypertension in Black people, more research is needed to investigate the intricate factors that potentiate hypertension.
In addition to the various factors that impact hypertension in Black adults, nonadherence to prescribed hypertension treatment, especially antihypertensive medication, is an important cause of uncontrolled BP. 8 It is estimated that 50%–80% of those diagnosed with hypertension do not take prescribed medication resulting in a lack of therapeutic effect. 9 Analysis of the National Health and Nutrition Examination Survey (NHANES) data also indicated that adherence to the Dietary Approaches to Stop Hypertension (DASH) eating plan has remained low over the past decade and continues to decrease. 10 , 11 Furthermore, Black adults are less likely to meet targeted physical activity guidelines than other racial/ethnic groups and females versus males have lower physical activity levels. 2 , 12 Better hypertension treatment adherence could improve not only BP control and cardiovascular health, but also decrease the need for high‐cost interventions and other expenses associated with frequent health care utilization. 13 Key to understanding these unique factors and problem‐solving adherence issues requires involvement of Black people in their plan of care as active participants in hypertension management with proper education, tools, and methods that inspire change. 14
Interventions that combine multifaceted approaches such as self‐management, digital technology, and interactive communication have been effective with BP control. 15 The Chronic Disease Self‐Management Program (CDSMP) developed by researchers at Stanford University has been successful in improving health and psychological outcomes. 16 This program addresses the effects of chronic diseases, such as hypertension, on the individual and skills needed to manage self‐care on a day‐to‐day basis in collaboration with treatment from the health care provider. 17 A detailed description of the CDSMP has been published elsewhere. 18 Only one study with Chinese adults conducted research with the CDSMP and hypertension, 19 leaving a dearth of research with Black adults.
To support methods to manage hypertension, advances in digital technology and mobile apps are increasingly used to communicate and track health metrics. Of the three pilot randomized controlled trials found, one used message apps 20 and two used technology with mobile apps in samples with majority Black men and women with hypertension. 21 , 22 Results from these studies have shown decreases in SBP, 22 increased medication adherence, 21 and non‐significant BP and medication adherence results. 20 Yet, there is limited research on digital health technology and Black adults diagnosed with hypertension.
Health coaching is an emerging intervention with interactive/two‐way communication that builds motivation, provides education, and promotes self‐management skills needed for healthy lifestyle behavior in individuals with chronic diseases. 23 Coaches use nonjudgmental dialogue to motivate goal setting while participants assume responsibility and are held accountable for goal attainment. 24 In studies with diverse racial/ethnic groups, coaching to improve BP control resulted in improved medication adherence and decreased BP, 25 lower BP and less visits to the health care provider, 26 and no significant difference in the BP of the two groups studied. 27 Only one study addressed hypertension and health coaching in a sample of Black adults with hypertension using a telephone‐based lifestyle coaching intervention. 28 Study findings revealed that coaching with culturally appropriate photographs, stories, and recipes for the DASH eating plan were more effective in improving BP control than usual care and enhanced pharmacotherapy monitoring. While promising, the literature is scarce on the effectiveness of health coaching as a means of BP management in Black adults.
Although research has been conducted with Black adults using various interventions, no known studies have implemented the CDSMP, interactive technology, and health coaching together in a sample of Black women with hypertension. With support, incorporating multiple strategies could enhance accountability, increase active engagement with self‐care management, empower change, and sustain BP control.
1.1. Purpose
The aim of this longitudinal pilot randomized controlled trial study was to examine the effects of Interactive Technology Enhanced Coaching (ITEC) versus Interactive Technology (IT) alone in achieving BP control, adherence to antihypertensive medication, and adherence to lifestyle modifications (physical activity, healthy diet, and weight management) post CDSMP over 3‐, 6‐, and 9‐months. Our primary outcome of interest was change in baseline SBP and DBP with BP control while the secondary outcomes of interest included an improved change in adherence to medication‐taking, physical activity (steps), dietary habits (calories/sodium), and weight management over time.
2. METHODS
2.1. Participants
A detailed account of the study methods and sample characteristics have been published elsewhere. 18 Ninety Black women in the Piedmont region of North Carolina were recruited from various community settings such as churches, sororities, and community organizations using flyers, social media, and/or face‐to‐face presentations. Individuals interested in the study provided contact information and were initially screened by telephone prior to meeting in‐person to determine BP eligibility. Individuals who self‐reported a normal BP were not eligible to participate in the study. Only those who self‐reported a BP ≥ to 130/80 had their BP manually measured by a trained registered nurse to verify study eligibility. All participants were informed about study details and consented to participate in the study prior to baseline data collection. Approval to conduct this study was obtained from the Institutional Review Board at The University of North Carolina at Charlotte.
Inclusion criteria included women who self‐identified as Black, English speaking, aged 18–70 (age when employment and career goals become better defined and then began to decline), 29 diagnosis of hypertension and currently on prescribed antihypertensive medication, SBP/DBP ≥ to 130/80, and owned a smartphone with home Wi‐Fi connectivity. Women were excluded if they reported a mental illness or uncontrolled/debilitating medical condition that interfered with daily functioning, unable to be physically active, current pregnancy, or SBP/DBP ≥160/100.
2.2. Procedure for data collection
The CDSMP and data collection procedure are described in full elsewhere. 18 To summarize, each study participant met weekly in groups of 10–16 for 6 weeks in a local church classroom to complete the CDSMP. Individual action plans or goals were consistent with the study aims. After completing the CDSMP, participants were randomly assigned to either the treatment or referent group. All participants continued to receive usual care for their hypertension.
Technology‐enhanced data were collected via the Fitbit Plus platform, a cloud‐based collaborative care platform designed to track and store data. All participants were provided with compatible tools and apps. Participant data were collected from three wireless tools (Omron BP monitor, Fitbit activity tracker, and Fitbit weight scale) and five apps (Omron Connect, Fitbit, MyFitnessPal, Apple Health if iPhone user or Google Fit if android user, and Fitbit Plus). A Fitbit account was set up for each participant using de‐identified names. To import data, apps were installed and synchronized to the participant's smartphone. The principal investigator oriented the treatment group to wireless tools and apps at month 1 and the referent group at month 4 using interactive learning with hands‐on practice, followed by almost 2 weeks of independent practice with assistance as needed. 18 At the completion of the study, participants were allowed to keep all tools and apps for continued self‐care management.
For out‐of‐office BP monitoring, ambulatory BP is the reference standard. 30 Research suggests that manual (auscultatory) BP taken according to American Heart Association recommendations by a well‐trained researcher is a strong predictor of ambulatory BP without white coat bias. 31 In this study, a trained registered nurse measured the BP with a Welch Allyn Tycos (DS58) hand aneroid sphygmomanometer using recommended BP measurement guidelines. 1 , 30 After individuals were seated for 5 min, three consecutive BP measurements were taken, 1 min apart and averaged to determine study eligibility. In addition to the baseline BP, weight was measured using a portable platform Seca 813 electronic scale and height was measured using a portable Seca 217 stadiometer. All details are reported elsewhere. 18 Blood pressure and weight were repeated during months 1–3 to compare the referent group with usual care to the treatment group with ITEC. No baseline data were collected for steps, medication adherence, or dietary intake (calories/sodium) in either group. For both groups, manual BP and weight measurements were obtained in‐person at 3‐, 6‐, and 9‐months to verify data from the wireless tools. In‐person visits provided an opportunity to monitor data accuracy, check equipment, troubleshoot problems, and provide a human element to the study.
With Fitbit Plus, each participant's data were tracked, analyzed, and transparently displayed providing opportunities for participants to better understand their condition and actively self‐manage their care. On the principal investigator's Fitbit platform, participant data were color coded to indicate adherence versus nonadherence to the action plan alerting the principal investigator to communicate with participants who were nonadherent. The principal investigator sent coaching messages (ITEC) through Fitbit Plus to the treatment group weekly for months 1–3 and bi‐weekly for months 4–6 with relevant individualized dialogue for goal attainment. During months 7–9, the treatment group was monitored with IT and no coaching to assess for sustainability. The referent group received usual care for months 1–3 and IT for months 4–6 and 7–9. Reminders were sent if they were not participating daily. Figure 1 presents an overview of the study design. Gift cards were given to participants for time expended for baseline assessment ($20) and repeated measures at 3‐ ($30), 6‐ ($40), and 9‐months ($60) totaling $150. 18
FIGURE 1.
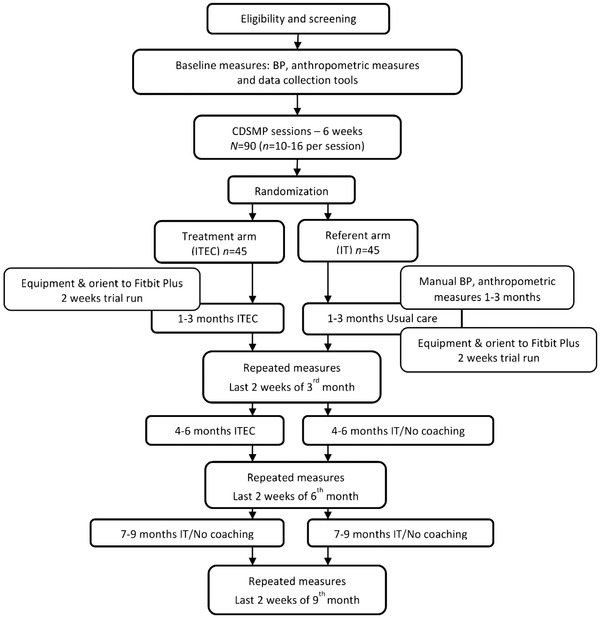
Overview of study design.
BP, blood pressure; CDSMP, Chronic Disease Self‐Management Program; ITEC, interactive technology enhanced coaching. Reprinted from “An interactive technology enhanced coaching intervention for Black women with hypertension: Randomized controlled trial study protocol,” by W. M. Abel and M. J. DeHaven, 2021, Research in Nursing & Health, 44(1), 24–36. Copyright 2020 by Wiley Periodicals LLC. Reprinted with permission.
2.3. Measurements
Tools and apps used to transmit data electronically to Fitbit Plus are briefly described below and details are reported elsewhere. 18 All data were automatically synchronized to smartphones with real‐time tracking in Fitbit Plus except for the manual insertion of BP data for android users and medication data for both android and iPhone users. Participants and the principal investigator were able to view all data on a continuous graph in Fitbit Plus.
2.3.1. Blood pressure
The Omron 10 wireless Bluetooth home BP monitor (Model BP786N) was used to collect BP data. Participants were trained to measure their BP twice per day (morning and night) according to American Heart Association guidelines for home BP monitoring. 32 , 33 Android users self‐reported their BP measurements in Fitbit Plus and emailed an automated Omron app BP spreadsheet weekly to the principal investigator to ensure accuracy of BP entries.
2.3.2. Physical activity tracker
The Fitbit Inspire HR was used to monitor physical activity by tracking the number of steps taken each day. Participants were trained to wear the Fitbit device daily during waking hours on the top of their non‐dominant wrist.
2.3.3. Weight
The Fitbit Aria 2 weight scale (Wi‐Fi Smart Scale) was used to measure weight in pounds. Participants were instructed to weight themselves daily, first thing in the morning after emptying their bladder.
2.3.4. Medication adherence
Prescribed anti‐hypertensive medications names, dosages, and time frequencies were input into Fitbit Plus by the principal investigator and updated if changes occurred. Participants self‐reported medication‐taking by manually checking the time they took their medication each day.
2.3.5. MyFitnessPal food diary
Using the MyFitnessPal food database, participants were instructed to self‐report their food intake after each meal or snack. MyFitnessPal app was used to track caloric/sodium intake.
2.4. Statistical analyses
Details of the statistical analyses plan are published elsewhere. 18 A sample size of 90 participants were recruited for random assignment to treatment and referent groups (n = 45 per arm) and measured at baseline and post CDSMP at 3‐, 6‐, and 9‐months. Our sample (N = 90) had approximately 80% power (assuming 5 dropouts per arm) to detect an effect size of ∼.205 or greater at the α = .05 level of statistical significance, given a realistic range of simulated unstructured covariance matrices. An 11% rate of attrition was anticipated to yield a final sample of 80 participants, an average of 40 per arm. Participants who dropped out of the study prior to receiving the allotted intervention were not included in the analysis.
The primary and secondary outcomes of interest were assessed by statistical analysis performed with a hierarchical, mixed‐effects repeated measures design. This model allowed for heterogeneous variances across time points. When appropriate a constrained longitudinal data analysis (cLDA) model was used, assuming the same baseline mean for treatment and referent arms with missing baseline data. The cLDA model examines treatment differences over time. Coffman et al 34 cite the cLDA as the method of choice for longitudinal randomized controlled trials because it yields robust estimates of treatment effect differences under reasoning data assumptions.
Both models are valid for repeated measures if data are missing at random (MAR). Little's test was used to determine if variables were missing completely at random (MCAR) while significant covariate‐dependent missing values were identified by regressing a dichotomous indicator variable for missingness against key models factors. 35 That is, if the missing values were non‐randomly associated with the latter variables of interest, the mechanism was deemed to be missing at random (MAR). A sampling of observed versus non‐unobserved items were subjectively scrutinized for signs of a ‘missing not at random (MNAR)’ pattern for the data. Overall, no relevant violation of the MAR assumption was evident in our analyses.
Descriptive statistics were used to summarize demographic data of participants. Rounding was based upon significant digits rather than a fixed number of decimal places (i.e., Goldilocks method). 36 Data were analyzed using SAS ‐ v 9.4 (Cary, NC).
3. RESULTS
Of the 83 Black women who completed the CDSMP, 77 had data in the Fitbit platform and were included in the analysis. Participants not included in the Fitbit analysis and the final sample were lost to follow‐up or withdrew from the study owing to inactivity. We estimated an 11% rate of attrition for each group of 45 which yielded 40 per group. 18 For the final sample of 69, the attrition rate was 18% (8 of 45) in the treatment group and 29% (13 of 45) in the referent group. The median age was 54 years and years diagnosed with hypertension was 10. Most (73%, n = 56) had completed an associate or higher degree and their weight was in the obese category (79%), n = 61. Many (73%, n = 56) worked full time and 55% (n = 42) had incomes ≥ $55 000 (Table 1).
TABLE 1.
Descriptive statistics, N = 77
| Participant characteristics | Median; IQR (Q3–Q1) or n (%) |
|---|---|
| Age | 54; 13 (61–49) |
| Years dx with hypertension | 10; 11 (15–4) |
| Blood pressure | |
| Systolic BP | 138; 15 (148‐132) |
| Diastolic BP | 83; 9 (88‐80) |
| Marital status | |
| Single (never married) | 21 (27) |
| Married | 34 (44) |
| Separated/divorced | 19 (25) |
| Widowed | 3 (4) |
| Education | |
| High school graduate | 2 (3) |
| Some comm. college | 11 (14) |
| Graduated comm. college | 6 (8) |
| Some 4‐year college | 8 (10) |
| Graduated 4‐year college | 27 (35) |
| Some graduate school | 1 (1) |
| Graduated graduate school | 22 (29) |
| Occupational status | |
| Work full‐time | 56 (73) |
| Retired with pension | 15 (20)a |
| Work part‐time/not employed | 9 (12) |
| Income | |
| < 10 000–24 999 | 10 (13) |
| 25 000–54 999 | 24 (31) |
| 55 000– > 100 000 | 42 (55) |
| Refused | 1 (1) |
| Health coverage | |
| Private | 58 (75) |
| Medicare | 13 (17) |
| Medicaid | 1 (1) |
| Free clinic | 2 (3) |
| No coverage | 3 (4) |
| Body Mass Index | |
| Normal weight (18.5–24.9 kg/m2) | 3 (4) |
| Overweight (25–29.9 kg/m2) | 13 (17) |
| Obesity (30 kg/m2 or >) | 61 (79) |
Table 1 reprinted from “An interactive technology enhanced coaching intervention for Black women with hypertension: Randomized controlled trial study protocol,” by W. M. Abel and M. J. DeHaven, 2021, Research in Nursing & Health, 44(1), 24–36. Copyright 2020 by Wiley Periodicals LLC. Reprinted with permission, License Number: 5414921100804. License date: October 23, 2022. Licensed Content Publisher: John Wiley and Sons.
Percentages may not add up to 100% because of rounding.
IQR, interquartile range; Q1, 25th quartile; Q3, 75th quartile. .
aWork full‐time = 1 and part‐time = 2.
Fitbit Plus captured participants completion of monitored activities that included BP, weight, physical activity (steps), and self‐reported medication adherence and food intake (calories/sodium). Participants used equipment and apps for daily monitoring. One or more of the monitored activities completed each day during the 36 weeks (252 days) for the treatment group and 24 weeks (168 days) for the referent group, were counted as one monitored day. Almost one‐third (31%, n = 24) of participants in both groups completed one or more monitored activities ≥80% of the monitored days. Participants with <20% completion of monitored activities withdrew from the study owing to inactivity (Table 2).
TABLE 2.
Completed ≥ 1 monitored activity each day
| Completion | 252 monitored days treatment group (n = 41) | 168 monitored days referent group (n = 36) | Total (N = 77) | ||
|---|---|---|---|---|---|
| % | n (%) | Range | n (%) | Range | n (%) |
| 80–100 | 13 (32) | 202–251 | 11 (31) | 135–167 | 24 (31) |
| 60– < 80 | 10 (24) | 152–201 | 3 (8) | 101–134 | 13 (17) |
| 40– < 60 | 8 (20) | 101–151 | 9 (25) | 68–100 | 17 (22) |
| 20– < 40 | 8 (20) | 51–100 | 10 (28) | 34–67 | 18 (23) |
| <20 | 2 (5) | 15–50 | 3 (8) | 2–33 | 5 (7) |
In Figures 2, 3, 4, the baseline data collected for BP and weight during months 1–3 compares the referent group with usual care to the treatment group (ITEC). As shown in Figures 5, 6, 7, 8, no baseline data was assessed for steps, medication adherence, or dietary intake (calories/sodium) for both groups. Thus, to compare the treatment and referent groups, the cLDA model was used to assume the same baseline mean and starting point for the treatment and referent arms with missing baseline data (Figures 5, 6, 7, 8).
FIGURE 2.
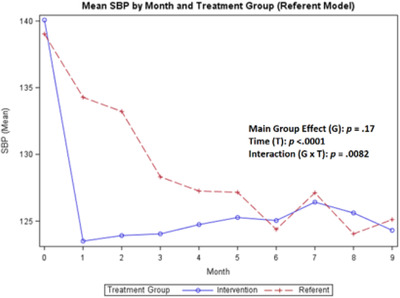
Mean systolic blood pressure
FIGURE 3.
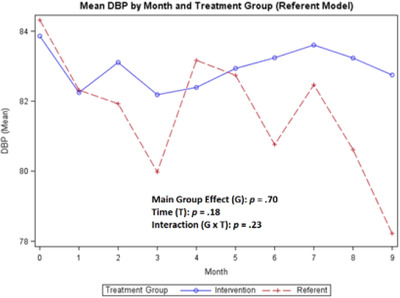
Mean diastolic blood pressure
FIGURE 4.
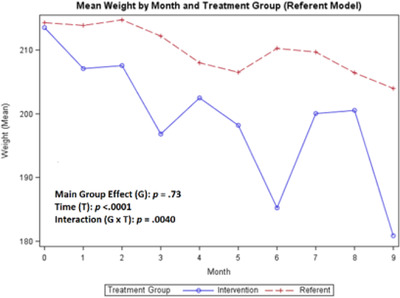
Mean weight
FIGURE 5.
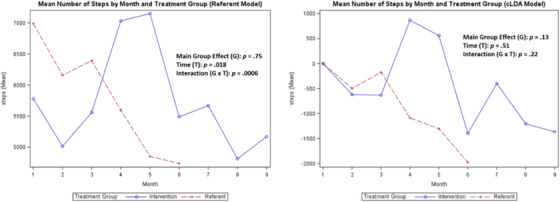
Mean number of daily steps
FIGURE 6.
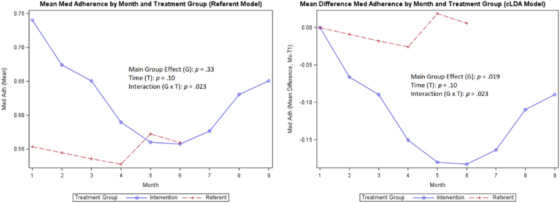
Mean medication adherence
FIGURE 7.
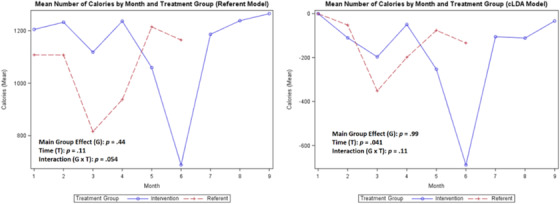
Mean calories
FIGURE 8.
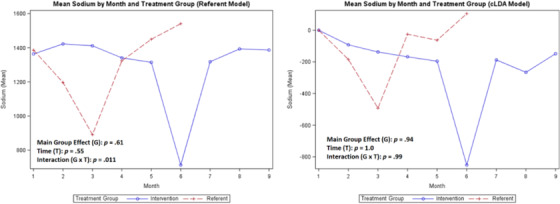
Mean sodium
A comparison of the coaching intervention on the means of each variable of interest (SBP, DBP, steps, weight, medication adherence, and calories/sodium) for the treatment group compared with the referent group is shown in Figures 2, 3, 4, 5, 6, 7, 8 with the main group effect (differences between the groups), main effect of time (differences between repeated measures), and the interaction effect (differences between groups over time). 37 The mean of each variable is shown by month in each figure for both groups.
For the primary outcome of change in SBP over time, the main group effect in the referent model showed no significant difference (p = .17) between the treatment and referent group, while the main effect of time indicated significant differences between repeated measures (p < .0001) and the interaction effect revealed differences over time (p = .0082). For the DBP, there was no significant difference in the main group effect (p = .70), main effect of time (p = .18), and the interaction effect (p = .23) (Figures 2, 3). The largest overall reduction in SBP occurred in the treatment group at month 1 and in the referent group around month 3 (Figure 2). The mean SBP decreased to <130 mmHg and the mean DBP decreased to <84 mmHg for both groups for the duration of the study with greater BP fluctuations in the referent group (Figure 3).
For the secondary outcomes, the main group effect in the referent model showed no significant difference between the treatment and referent group for all study variables; and in the cLDA model, only medication adherence (p = .019) was significant. In the referent model, the main effect of time indicated significant differences between repeated measures for weight (p < .0001) and steps (p = .018); and in the cLDA model, only calories (p = .041) were significant. In the referent model, an interaction effect revealed differences over time and was significant for all measures; and in the cLDA model, only medication adherence (p = .023) was significant (see Figures 4, 5, 6, 7, 8). The mean weight loss was greater in the treatment group with fluctuating lines from baseline to month 9 (Figure 4). Although expected to increase, the mean number of steps per day used to reflect physical activity gradually decreased with fluctuating lines in the treatment group. The sharpest decrease in physical activity (steps) was noted during month 5 and remained low through month 9 (Figure 5) indicating the cooler season of November through February. Self‐reported medication adherence did not reach the standard adherence level of ≥80% for either group during the study period and was lower in the referent group at month 9 (Figure 6). Of all the self‐monitoring activities, inputting food intake into MyFitnessPal after each meal had the lowest daily participant engagement as reflected in the low means for calorie/sodium intake. As physical activity (steps) decreased, dietary intake (calories/sodium) increased in months 6–9. (Figures 5, 7, and 8).
In Figures 2, 3, 4, 5, 6, 7, 8, the term non‐monotonic describes the fluctuating lines that go up and down at different time points. The level of participation among participants in both groups was noted to be lower around Labor Day (month 4), the Thanksgiving holiday (month 6), and toward the end of the study (month 9). Because of the relatively small sample size, we were unable to adjust the model for a seasonality effect. Mean averages during those times may be affected by low participation rates that contributed to the non‐monotone missing data pattern.
Also, the non‐monotonicity ran parallel to the coaching intervention in the treatment group. As coaching began to decrease in months 4–6 and was discontinued in months 7–9, SBP and DBP began to increase, number of steps decreased, weight increased, medication adherence decreased, and calories/sodium increased (Figures 2, 3, 4, 5, 6, 7, 8). This pattern was not observed in the referent group.
4. DISCUSSION
This 9‐month pilot randomized controlled trial study evaluated the effectiveness of a coaching intervention in improving BP, weight, dietary intake, physical activity, and medication adherence as participants self‐monitored using interactive technology. Preliminary evidence supports the feasibility of this longitudinal coaching and technology intervention that was effectively implemented in Black women with hypertension using self‐care management strategies.
Unlike disadvantaged populations, most Black women in this study were well‐educated with adequate incomes and health insurance. However, the similarities these women share with disadvantaged populations is often overlooked and includes chronic stress that impacts their ability to implement and sustain changes necessary for positive health behaviors. 38 Future research should develop effective approaches to manage and reduce stress in this population with aims to decrease its impact on healthy behaviors.
The CDSMP program had a positive effect on decreasing and maintaining SBP control in both groups to <130 mmHg. This decrease in SBP occurred one month after the CDSMP for the treatment group, and by month 3 in the referent group. In both groups, DBP decreased around month 1 and remained <84 mmHg throughout the study. Only the SBP was significant for the main effect of time and the interaction effect. The DBP was lower in the treatment group. In contrast, a study conducted by Zhang and colleagues 19 using a pre‐post‐study design with the CDSMP showed no difference in the SBP between the treatment and referent group and the DBP was higher in the treatment group.
Weight decreased more in the treatment than the referent group. Most participants in this study were obese with a higher socioeconomic status. Similarly, in another study, 39 Black adults with a college education and higher incomes had a greater obesity burden. Higher socioeconomic status for Black adults could result in higher stress levels related to racism, discrimination, segregation/concentration, 40 , 41 decreased social support networks, pressure to provide financial support to extended family, and increased effort to fit into “White spaces. 41 ” Over time, these stressors may contribute to poor physical and mental health outcomes for Black adults. 41 Steps decreased instead of increasing during the study period. Decreased steps were associated with cooler weather and the holidays (Thanksgiving and Christmas). Reasons for physical inactivity may include life events taking priority, lack of motivation, and unsafe neighborhood environments. Medication adherence numbers decreased, and caloric/sodium means were low. Both of these activities required participants to manually input data daily. It is possible that participants forgot or chose not to record the data daily because it was an additional task added to an already busy schedule.
Some statistical differences in the main group effect, main effect of time, and interaction effect may have resulted from changes in scores over time owing to the low participation rates. Accordingly, the non‐monotonicity seen in the figures reflect low participation in study activities. Also, the influence of the coaching intervention in the treatment group cannot be discounted. As coaching decreased, treatment group participant engagement with activities declined. Thus, health coaching to improve BP control may be an effective tool for hypertension self‐management.
During a CDSMP class session, a question emerged that echoed the views of other women in the study, “Why do we not hear this information (on self‐care management) from our health care providers?” Although the majority of women in this study were well‐educated with an associate or higher degree, education alone does not necessarily equate to behavior change. 42 While the CDSMP provided training and skills necessary to increase confidence for self‐care management, completion of the daily monitored activities in the study were absent in over half of the participants in both groups who completed one or more monitored activities <60% of the time. We recognize that monitoring five daily activities necessitated making changes in daily routines. Consequently, some participants may have been overwhelmed with integrating and prioritizing the study activities with daily life events. As for others, motivation to change behavior over time may have been lacking. Thus, future studies could allow participants to select the number of goals they would like to work on in an effort to increase motivation to track self‐care behaviors. In addition, methods could be instituted to identify and assist those who may be overburdened by the dictates of making lifestyle changes while balancing day‐to‐day demands. Also, exploring motivation and readiness to make self‐care management a priority deserves further exploration.
The main strength of this longitudinal study was the ability to compare groups and collect objective data from digital tools that could be viewed in real‐time by both the participant and the researcher. The use of technology allowed participants to track their health data and be actively engaged in self‐management. In addition, we were able to observe both groups for sustainable behavior change. Participants who were motivated and committed to set realistic goals and carry out their action plans were more likely to succeed with self‐care management lifestyle changes.
Several limitations were noted for this study. Baseline data were not collected for several study measures (physical activity, medication adherence, and dietary intake) and this prevented the capture of progress from the same reference point for both groups. The cLDA model was used to assume a baseline mean for both arms. Participation in five goals (BP control, medication adherence, physical activity, weight management, and healthy food intake) simultaneously may have hindered overall success. Asking participants what they want to change and achieving one goal before proceeding to the next may be a better future strategy. Also, medication adherence and food intake were self‐reported, underreported, and subject to recall bias. In this study, the referent group was only contacted if they were not engaging in daily activities, whereas the treatment group had planned weekly and bi‐weekly coaching interventions. To minimize attrition in future studies, the referent group should receive the same amount of interpersonal communication as the treatment group but not related to the study intervention. Participant engagement decreased after the weekly CDSMP classes ended. The social support during the classes, empowered participants to share experiences, problem‐solve with each other, and commit to achieving goals while holding each other accountable for progress, was no longer available. Social support among Black women has been shown to promote healthy lifestyle changes 43 and could be used as a future intervention strategy. Lastly, this study was conducted with a sample of highly educated Black women, which limits the ability to generalize the results to all Black women with hypertension.
5. CONCLUSION
The prevalence of hypertension in Black women is daunting when considering the magnitude of hypertension‐related diseases and their adverse effects. Methods such as the CDSMP, interactive technology, and coaching may help Black women prioritize self‐care management in the context of their everyday environment. Enhancing these methods to solicit commitment to the hypertension treatment regimen warrants further exploration in this population.
AUTHOR CONTRIBUTIONS
Willie M. Abel and Mark DeHaven conceived the concept and all authors had input into the study design. Willie M. Abel collected the data. Jimmy Efird completed the statistical data analysis. All authors discussed the results and contributed to writing the manuscript. The final manuscript was read and approved by all authors.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
PATIENT CONSENT STATEMENT
All participants were informed about study details and consented to participate in the study prior to baseline data collection.
ACKNOWLEDGMENTS
The authors thank the women who agreed to participate in this study. Funding source was the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K01HL140288. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the United States Department of Veterans Affairs.
Abel WM, Efird JT, Crane PB, Ferdinand KC, Foy CG, DeHaven MJ. Use of coaching and technology to improve blood pressure control in Black women with hypertension: Pilot randomized controlled trial study. J Clin Hypertens. 2023;25:95–105. 10.1111/jch.14617
Clinical Trial Registration: ClinicalTrials.gov Identifier NCT0357799.
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13‐e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart association. Circulation. 2022;145(8):e153‐e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. doi: 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;379(10292):2385‐2438. [DOI] [PubMed] [Google Scholar]
- 5. Murphy SL, Xu J, Kochanek KD, Arias E, Tejada‐Vera B. Deaths: final data for 2018. Natl Vital Stat Rep. 2021;69(13):1‐83. [PubMed] [Google Scholar]
- 6. Maraboto C, Ferdinand KC. Update on hypertension in African‐Americans. Prog Cardiovasc Dis. 2020;63(1):33‐39. doi: 10.1016/j.pcad.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 7. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart association. Circulation. 2017;136(21):e393‐e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 8. Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta‐analysis. Medicine. 2017;96(4):e5641. 10.1097/MD.0000000000005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53‐e90. doi: 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim H, Andrade FC. Diagnostic status of hypertension on the adherence to the dietary approaches to stop hypertension (DASH) diet. Prev Med Rep. 2016;4:525‐531. doi: 10.1016/j.pmedr.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinberg D, Bennett GG, Svetkey L. The DASH diet, 20 years later. JAMA. 2017;317(15):1529‐1530. doi: 10.1001/jama.2017.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams WM, Yore MM, Whitt‐Glover MC. Estimating physical activity trends among blacks in the United States through examination of four national surveys. AIMS Public Health. 2018;5(2):144‐157. doi: 10.3934/publichealth.2018.2.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124‐1140. doi: 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 14. Crittenden D, Seibenhener S, Hamilton B. Health coaching and the management of hypertension. J Nurse Pract. 2017;13(5):e237‐e239. [Google Scholar]
- 15. Li R, Liang N, Bu F, Hesketh T. The effectiveness of self‐management of hypertension in adults using mobile health: systematic review and meta‐analysis. JMIR Mhealth Uhealth. 2020;8(3):e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lorig K, Chronic disease self‐management program: insights from the eye of the storm. Front Public Health. 2015;2:253. doi: 10.3389/fpubh.2014.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorig K, Laurent D, Gonzalez V, Sobel D, Minor M, Gecht‐Silver M. Living a Healthy Life with Chronic Conditions. 5th ed. Bull Publishing Company; 2020. [Google Scholar]
- 18. Abel WM, DeHaven MJ. An interactive technology enhanced coaching intervention for Black women with hypertension: randomized controlled trial study protocol. Res Nurs Health. 2021;44(1):24‐36. doi: 10.1002/nur.22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Liu S, Sheng X, Lou J, Fu H, Sun X. Evaluation of a community‐based hypertension self‐management model with general practitioners. Int J Health Plann Manage. 2019;34(3):960‐974. doi: 10.1002/hpm.2867 [DOI] [PubMed] [Google Scholar]
- 20. Buis L, Hirzel L, Dawood RM, et al. Text messaging to improve hypertension medication adherence in African Americans from primary care and emergency department settings: results from two randomized feasibility studies. JMIR Mhealth Uhealth. 2017;5(2):e9. doi: 10.2196/mhealth.6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Still CH, Margevicius S, Harwell C, et al. A community and technology‐based approach for hypertension self‐management (COACHMAN) to improve blood pressure control in African Americans: results from a pilot study. Patient Prefer Adherence. 2020;14:2301‐2313. doi: 10.2147/PPA.S283086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zha P, Qureshi R, Porter S, et al. Utilizing a mobile health intervention to manage hypertension in an underserved community. West J Nurs Res. 2020;42(3):201‐209. doi: 10.1177/0193945919847937 [DOI] [PubMed] [Google Scholar]
- 23. Conn S, Curtain S. Health coaching as a lifestyle medicine process in primary care. Aust J Gen Pract. 2019;48(10):677‐680. [DOI] [PubMed] [Google Scholar]
- 24. Sforzo GA, Kaye MP, Todorova I, et al. Compendium of the health and wellness coaching literature. Am J Lifestyle Med. 2017;12(6):436‐447. doi: 10.1177/1559827617708562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu JR, Cummings DM, Li Q, et al. The effect of a practice‐based multicomponent intervention that includes health coaching on medication adherence and blood pressure control in rural primary care. J Clin Hypertens. 2018;20(4):757‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margolius D, Bodenheimer T, Bennett H, et al. Health coaching to improve hypertension treatment in a low‐income, minority population. Ann Fam Med. 2012;10(3):199‐205. doi: 10.1370/afm.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Persell SD, Peprah YA, Lipiszko D, et al. Effect of home blood pressure monitoring via a smartphone hypertension coaching application or tracking application on adults with uncontrolled hypertension: a randomized clinical trial. JAMA Network Open. 2020;3(3):e200255. doi: 10.1001/jamanetworkopen.2020.0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen‐Huynh MN, Young JD, Ovbiagele B, et al. Effect of lifestyle coaching or enhanced pharmacotherapy on blood pressure control among Black adults with persistent uncontrolled hypertension: a cluster randomized clinical trial. JAMA Netw Open. 2022;5(5):e2212397. doi: 10.1001/jamanetworkopen.2022.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James SA. The John Henryism scale for active coping. In: Jones RL, ed. Handbook of Tests and Measurements for Black Populations. Cobb & Henry Publishers; 1996:415‐425. [Google Scholar]
- 30. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart association. Hypertension. 2019;73(5):e35‐e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Espinosa R, Spruill TM, Zawadzki MJ, et al. Can blood pressure measurements taken in the physician's office avoid the ‘white coat’ bias? Blood Press Monit. 2011;16(5):231‐237. doi: 10.1097/MBP.0b013e32834b45d2 [DOI] [PubMed] [Google Scholar]
- 32. American Heart Associaiton Editorial Staff . Monitoring your blood pressure at home. https://www.heart.org/en/health‐topics/high‐blood‐pressure/understanding‐blood‐pressure‐readings/monitoring‐your‐blood‐pressure‐at‐home
- 33. Shimbo D, Artinian NT, Basile JN, et al. Self‐measured blood pressure monitoring at home: a joint policy statement from the American Heart association and American Medical association. Circulation. 2020;142(4):e42‐e63. doi: 10.1161/CIR.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 34. Coffman CJ, Edelman D, Woolson RF. To condition or not condition? Analysing ‘change’ in longitudinal randomised controlled trials. BMJ Open. 2016;6(12):e013096. doi: 10.1136/bmjopen-2016-013096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li C. Little's test of missing completely at random. Stata J. 2013;13(4):795‐809. [Google Scholar]
- 36. Efird JT. Goldilocks rounding: achieving balance between accuracy and parsimony in the reporting of relative effect estimates. Cancer Inform. 2021;20:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Statistics Solutions . Statistical Interaction: More Than the Sum of its Parts. Complete Dissertation. https://www.statisticssolutions.com/statistical‐interaction‐more‐than‐the‐sum‐of‐its‐parts/
- 38. Kalinowski J, Kaur K, Newsome‐Garcia V, et al. Stress interventions and hypertension in Black women. Womens Health. 2021;17:17455065211009751. doi: 10.1177/17455065211009751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bell CN, Thorpe RJ Jr, Bowie JV, LaVeist TA. Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol. 2018;28(3):147‐152. doi: 10.1016/j.annepidem.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 40. Reardon SF, Fox L, Townsend J. Neighborhood income composition by household race and income, 1990–2009. Ann Am Acad Pol Soc Sci. 2015;660(1):78‐97. [Google Scholar]
- 41. Hudson D, Sacks T, Irani K, Asher A. The price of the ticket: health costs of upward mobility among African Americans. Int J Environ Res Public Health. 2020;17(4):1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arlinghaus KR, Johnston CA. Advocating for behavior change with education. Am J Lifestyle Med. 2018;12(2):113‐116. doi: 10.1177/1559827617745479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tkatch R, Musich S, Draklellis J, et al. Claim more™: empowering African American women to make healthy choices. J Holist Nurs. 2018;36(1):91‐100. doi: 10.1177/0898010117691167 [DOI] [PMC free article] [PubMed] [Google Scholar]


