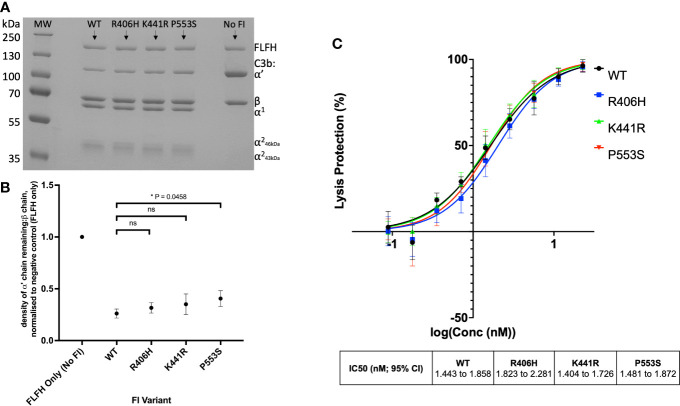
Figure 7.
Characterisation of AMD –linked CFI Variants. (A) Fluid phase cofactor assays for FI variants. Separation of C3b products by SDS-PAGE followed by Coomassie staining was used to reveal the α’, β and α1 chains of C3b. (B) Densitometry analysis of C3b breakdown by each FI variant. The mean (+/- SD) density of the normalised α’-chain present in the products of 3 fluid phase reactions is plotted for each FI variant. The density of the α’-chain was given as a ratio of the β-chain density before being normalised to a negative control (with no FI) to give a relative density of the α’-chain remaining for each reaction. Higher (closer to 1) α’–chain remaining suggests reduced cofactor activity of the FI variants. Fluid phase assays were repeated 3 times and a standard t test was used to compare mean normalised α’-chain remaining values for each variant vs the WT FLFH = full-lenght factor H. *P < 0.05. ns = non-significant. (C) Haemoltyic assays of CA for FI variants. Fi variants were titrated through C3b-coated SRBCs before the AP C3 convertase was built on any C3b remaining on the cell surfaces. Cells were lysed with Guinea pig serum in an FI concentration dependent manner with a readout of OD at 412nm. The efficacy of each FI variant was calculated using a non-linear 4-parameter fit curve after normalization to no FI (100%) and 0% (buffer only) lysis controls and IC50s are given with 95% CIs. Each plotted point is % protection from lysis compared to the 0% lysis control and bars represent SD for each point calculated from 3 experimental repeats run in duplicate.