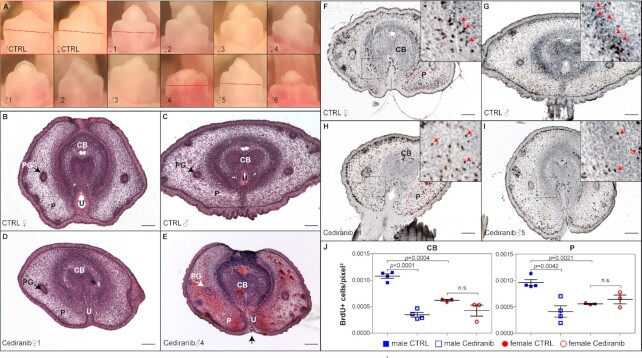
Fig. 5.
Adverse effect of Cediranib on male GT development in vivo. (A) Gross morphology of control E17.5 GTs (red outline), and that of a litter of 10 pups treated daily with Cediranib from E14.5 to E16.5. Red lines indicate planes of sections shown in B to I. (B to E) H&E staining of transverse sections of control- and Cediranib-treated E17.5 GT. PG, preputial glands; P, prepuce; U, urethra. (F to I) BrdU staining of transverse sections of control- and Cediranib-treated GT. Insets showed magnified view of boxed areas. Red arrows point to BrdU+ cells. (J) Quantification of BrdU+ cells per unit area in corpus body (white-dotted regions in F and H) and prepuce (red-dotted regions in F an H). CB, CTRL male 0.001079 ± 4.346e-005, N = 4 vs. Cediranib male 0.0003480 ± 4.444e-005, N = 4, P < 0.0001; CTRL female 0.0006211 ± 2.044e-005 N = 3, vs. Cediranib female 0.0004279 ± 0.0001056, N = 3, P = 0.1467; P, CTRL male 0.0009609 ± 5.790e-005 vs. Cediranib male 0.0004110 ± 0.0001081, N = 4, P = 0.0042; CTRL female 0.0009609 ± 5.790e-005, N = 4 vs. Cediranib female 0.0005567 ± 1.158e-005, N = 3, P = 0.14.