Abstract
Background
Acinetobacter baumannii is a pathogen responsible for nosocomial infections, especially in patients with burns and ventilator‐associated pneumonia (VAP). The aims of this study was to compare the biofilm formation capacity, antimicrobial resistance patterns and molecular typing based on PFGE (Pulsed‐Field Gel Electrophoresis) in A. baumannii isolated from burn and VAP patients.
Materials and Methods
A total of 50 A. baumannii isolates were obtained from burn and VAP patients. In this study, we assessed antimicrobial susceptibility, biofilm formation capacity, PFGE fingerprinting, and the distribution of biofilm‐related genes (csuD, csuE, ptk, ataA, and ompA).
Results
Overall, 74% of the strains were multidrug resistant (MDR), and 26% were extensively drug‐resistant (XDR). Regarding biofilm formation capacity, 52%, 36%, and 12% of the isolates were strong, moderate, and weak biofilm producers. Strong biofilm formation capacity significantly correlated with XDR phenotype (12/13, 92.3%). All the isolates harbored at least one biofilm‐related gene. The most prevalent gene was csuD (98%), followed by ptk (90%), ataA (88%), ompA (86%), and csuE (86%). Harboring all the biofilm‐related genes was significantly associated with XDR phenotype. Finally, PFGE clustering revealed 6 clusters, among which cluster No. 2 showed a significant correlation with strong biofilm formation and XDR phenotype.
Conclusion
Our findings revealed the variable distribution of biofilm‐related genes among MDR and XDR A. baumannii isolates from burn and VAP patients. A significant correlation was found between strong biofilm formation capacity and XDR phenotype. Finally, our results suggested that XDR phenotype was predominant among strong‐biofilm producer A. baumannii in our region.
Keywords: Acinetobacter baumannii, antimicrobial resistance, biofilm, biofilm‐related genes, PFGE
A. baumannii strains isolate from burn and VAP patients. we assessed antibiotic susceptibility, biofilm formation capacity, PFGE fingerprinting, and the distribution of biofilm‐related genes. Overall, 74% of the strains were multidrug resistant (MDR), and 26% were extensively drug‐resistant (XDR). All the isolates harbored at least one biofilm‐related gene. Finally, PFGE clustering revealed 18 clusters, among which cluster No. 2 showed a significant correlation with strong biofilm formation and XDR phenotype.

1. INTRODUCTION
Burnt skin provides a suitable environment for colonization and proliferation of bacteria. Patients with burns and ventilator are two groups at high risk for bacterial infections. In these patients, Acinetobacter baumannii (A. baumannii) can be transmitted by invasive clinical procedures, such as mechanical ventilation, and indwelling devices. 1 Multidrug‐resistant (MDR) A. baumannii is an important ubiquitous pathogen responsible for a variety of community and hospital infections and forms biofilms in healthcare settings. 2 Eradicating A. baumannii faces dramatic problems due to antimicrobial therapy failure secondary to the emergence of MDR and extensively‐drug resistant (XDR) isolates. In fact, antimicrobial resistance is a great threat increasing A. baumannii‐related morbidity and mortality, 3 and biofilm formation provides the driving force for the emergence of new and more antimicrobial resistant phenotypes, which are more strongly associated with nosocomial infections. 4
Although A. baumannii is naturally resistant to many available antibacterial agents, the development of antimicrobial resistance against other generation antimicrobials such as carbapenems, leading to antimicrobial therapy failure, highlights the importance of the infections caused by these bacteria as a significant health problem. 3 Biofilm formation is a main virulence factor and a hallmark characteristic of this bacterium. Acinetobacter spp. can form biofilm at solid–liquid and air‐liquid interface. The biofilm formation rate in A. baumannii at the solid–liquid interface is higher than other Acinetobacter species. 5 Within biofilm, A. baumannii can acquire genes encoding antimicrobial resistance from other bacteria through mobile genetic elements including plasmids, integrons, or transposons. 3
Biofilm formation and antimicrobial resistance have been found to be directly correlated in A. baumannii isolates, suggesting that biofilm formation is a necessary step in the development of MDR bacteria. 4 , 6
Multidrug resistant and XDR A. baumannii are commonly found in healthcare‐associated infections, generally in the context of nosocomial infections. Multidrug resistance profile as defined by the isolate being non‐susceptible to at least one agent in ≥3 antimicrobial categories. Isolates of A. baumannii with resistance to at least one agent in all but two or fewer antimicrobial categories were considered XDR. 7
It has been proven that several factors are associated with biofilm‐related genes, and in fact, biofilm formation largely governs the severity of infections and triggers antimicrobial resistance. For example, in catheter‐related bacteremia and aspiration pneumonia, the prevalence of A. baumannii harboring biofilm‐related genes, including ompA, ataA, csuA, csuE, and ptk, was reported to be high among antimicrobial ‐resistant strains. 8 The outer membrane protein A (OmpA), a 38‐kDa protein of A. baumannii, is encoded by the ompA gene and acts as a major porin that allows for biofilm formation on biotic surfaces, such as epithelial cells, through facilitating porin/fibronectin interactions. 9
A. baumannii is generally non‐motile; however, it possesses several genes, known as chaperone‐usher pilus (csuA/BABCDE) assembly operon, that are required to assemble pilus to produce strong biofilm on polystyrene and glass surfaces such as catheter and ventilators. 2 Interestingly, biofilm maturation is promoted by csu‐operon, and the absence of the cusE gene results in the lack of pilus production, disrupting biofilm formation. 10 A. baumannii colonization is influenced by the presence of the acinetobacter trimeric autotransporter adhesion (ata) gene that contributes to adhesion to and invading human endothelial and epithelial cells. 11 Besides, the ata gene has a wide variety of molecular activities and participates in most biological processes such as adhesion, biofilm formation, immune evasion, angiogenesis, and apoptosis. On the other hand, Ptk is a putative protein tyrosine kinase encoded by the ptk gene, required for capsule polymerization. This is without a doubt one of the most important factors to promote biofilm formation by A. baumannii. 9 Based on population genetic studies and epidemiological investigations of A. baumannii, there are several typing methods, including multilocus sequence typing (MLST), pulsed‐field gel electrophoresis (PFGE), multiple‐locus variable number tandem repeats (VNTRs) analysis (MLVA), and whole genome sequencing (WGS). 12 , 13 Among these methods, PFGE is considered the gold standard due to its sensitivity, reproducibility and discriminatory power, and to determine the prevalence of pathogens within and between hospitals and their stability in the environment are used. 14 In this study, we aimed to investigate the presence of biofilm‐related genes (ompA, csuA, csuE, ptk, and ataA) and their association with biofilm formation and perform molecular typing based on PFGE in A. baumannii isolated from burn and VAP patients.
2. MATERIALS AND METHODS
2.1. Study population and bacterial isolates
This cross‐sectional study was in accordance with the Declaration of Helsinki (between October 2020 and July 2021). All samples were collected from two hospitals in Tehran (Rasool Akram and Shahid Motahhari). Informed consent and ethical approval were obtained from the hospitals' authorities and the institutional ethics committee, respectively, prior to the study. Non‐replicating A. baumannii bacteria were collected from burn and VAP patients. Primary identification A. baumannii isolates was based on the Gram staining reaction and colony morphology. Standard biochemical tests such as catalase, citrate, triple sugar iron agar, urease test, oxidase, methyl red, Voges Prausker, and indole production were used to identify the A. baumannii isolates. 15 All the isolates were confirmed using molecular (gyrB) 16 and bacteriological identification tests (API 20NE).
2.2. Antimicrobial ausceptibility testing
Antibacterial susceptibility patterns were assessed using the disk‐agar diffusion method, applying piperacillin‐tazobactam (100/10 μg), ampicillin/sulbactam (10/10 μg), imipenem (10 μg), meropenem (10 μg), ceftazidime (30 μg), cefepime (30 μg), ceftriaxone (30 μg) gentamicin (10 μg), and ticarcillin‐clavulanic acid (75/10 μg) (Himedia) antimicrobials. Minimum inhibitory concentrations (MIC) of polymyxin B and colistin were determined by the E‐test method (AB BIODISK), and results were interpreted using Clinical and Laboratory Standards Institute 2020 (CLSI, 2020) guidelines. 17 All breakpoints were available for the antibacterial agents. Escherichia coli ATCC 25922, E. coli ATCC 35218, and Pseudomonas aeruginosa ATCC 27853 were used as internal controls. MDR isolates of A. baumannii exhibit resistance to at least one agent from three antimicrobial classes, whereas XDR isolates of A. baumannii exhibit resistance to at least one agent from all, but two or fewer antimicrobial categories. PDR‐A. baumannii isolates were non‐susceptible to all antimicrobial agents. 18
2.3. Biofilm formation
Biofilm formation was assessed using the crystal violet quantification test. Briefly, isolates were inoculated in the LB broth culture medium (Conda) and incubated at 37°C for 24 h. The bacterial concentration was then measured by a spectrophotometer at 650 nm (OD = 0.1–0.08). The bacterial suspension (190 μl LB medium + 10 μl cultured bacteria) was poured into each well of a 96‐well microplate and incubated at 37°C for 24–48 h. The biofilm formation assay was carried out three times for each sample, and the LB medium was used as a negative control in all experiments. Planktonic cells were removed, and after three times of washing with PBS, biofilm plates were fixed with 150 μl of 99% v/v methanol (Merck), and then each well was stained with crystal violet (1%, w/v) and incubated at room temperature for 20 min. 19
Biofilm was decolorized by ethanol‐acetone 33% (80, 20, v/v) for 20 min, and the supernatant was collected. Lastly, the absorbance was measured at 595 nm, and biofilm production capacity was quantified by calculating a score based on OD595 and categorized as no (OD < optical density cutoff value, ODc, −), weak (ODC < OD ≤2ODC, +), moderate (2ODc < OD ≤3ODc, + +), and strong (OD > 3ODc) biofilm formation. For the evaluation of biofilm formation, Mueller Hinton Broth (MHB) and A. baumannii ATCC 19606 were used as negative and positive controls, respectively. Triplicates of all experiments were conducted.
2.4. Identifying biofilm‐related genes
Whole DNA was extracted from all samples by boiling. 20 Biofilm‐related genes, including ompA, csuA, csuE, ptk, and ataA were amplified utilizing specific primers listed in Table 1. The PCR reaction was performed at the final volume of 25 μl, containing 1× PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTP mix, 10 pmol of each primer, and 50 ng of template DNA. PCR mixtures were subjected to the following thermal cycling: 5 min at 94°C, followed by 35 cycles with denaturation at 94°C for 50 s, annealing at 55°C–57°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 5 min.
TABLE 1.
Primers list was usded in this study
| Genes | Primers | Sequences 5′–3′ | Weight (bp) | References |
|---|---|---|---|---|
| ataA | ataA‐F | ACGACTATCAACATTTTTAAGCTGG | 101 | In this study |
| ataA‐R | TTGGGTCGGCTGGAAAAGAA | |||
| csuD | csuD‐F | ATACCGACCTTTCACGGCTG | 335 | In this study |
| csuD‐R | GCCAGTATCGCCCTGCTTAT | |||
| csuE | csuE‐1F | CTTTAGCAAACATGACCTACC | 702 | 51 |
| csuE‐1R | TACACCCGGGTTAATCGT | |||
| csuE‐2F | GGCGAACATGACCTATTT | 580 | ||
| csuE‐2R | CTTCATGGCTCGTTGGTT | |||
| ompA | ompA‐1F | GATGGCGTAAATCGTGGTA | 355 | 51 |
| ompA‐1R | CAACTTTAGCGATTTCTGG | |||
| ompA‐2F | GACCTTTCTTATCACAACGA | 343 | ||
| ompA‐2R | CAACTTTAGCGATTTCTGG | |||
| ptk | ptk‐F | AGCCATAACCATAGCCAGCG | 465 | In this study |
| ptk‐R | ACTCGTGGTAAGAGCCCAAC |
Note: The primers for ataA, csuD and ptk were designed using Gene Runner (Version 3.05, Hastings Software).
2.5. Pulsed field gel electrophoresis genotyping
Genetic relatedness of collected isolates was carried out by Pulsed‐Field Gel Electrophoresis (PFGE) as described previously. 21 Briefly, all pure‐cultured isolates were embedded in agarose plugs and then treated with a cell suspension buffer (CSB) containing EDTA and proteinase K (20 mg. mL−1). The plaques were thoroughly washed and then digested by the Apa I restriction enzyme (TaKaRa, Dalian) at 20°C for 5 h. All PFGE samples were loaded into the CHEF‐DR III system (Bio‐Rad) under the condition described by Qi et al. 22
Finally, PFGE patterns were analyzed and processed by the GelCompare II system (Applied Maths, Sint‐Martens‐Latem), and a genetic similarity dendrogram was generated based on the UPGMA algorithm with a position tolerance of 1.5%. The genetic relatedness was determined according to the criteria described by Tenover et al. 23 and PFGE patterns were distinguished at a similarity cutoff of 80%.
2.6. Statistical analysis
Statistical analysis was performed in SPSS version 20 software (SPSS, Inc.) and GraphPad Prism version 8 software (GraphPad Software Inc.). The Chi‐square test and Fisher's exact test were used to determine statistically significant associations between main variables at a p value of <0.05.
3. RESULTS
3.1. Population and antimicrobial susceptibility
A. baumannii isolates were identified by various tests that included: Gram‐negative coccobacilli, catalase positive, urease negative, H2S negative, oxidase negative, gas negative, citrate positive, indole negative, Voges‐Proskauer negative and methyl red positive. Isolates of A. baumannii were collected from burn patients (23/50) and patients with VAP (27/50), of whom 36 (72%) were male and 14 (28%) were female, with a mean age of 44.9 ± 12 years (range: 10–75 years). Overall, 74% (37/50) were MDR and 26% (13/50) were XDR. All the isolates were intermediate to colistin (MICs range from 0. 25 to 1 μg/ml) and polymyxin‐B. (MICs range from 0. 5 to 2 μg/ml). Resistance to meropenem was the most common observation (92%, 46/50) while the least common resistance was related to ampicilin/sulbactam (42%, 21/50). The resistance rates against gentamicin, imipenem, cefepime, ceftazidime, ceftriaxone, piperacillin/tazobactam, and ticarcillin/clavulanic acid were 44% (22/50), 88% (44/50), 86% (43/50), 88% (44/50), 84% (42/50), 86% (43/50), and 66% (33/50), respectively.
There was no statistically significant difference in the antimicrobial resistance rate between the bacteria isolated from burn or VAP patients (p = 0.1). The prevalence of XDR in the isolates from burn and VAP patients was 39.13% (9/23) and 14.81% (4/27), respectively. No significant association was found between XDR phenotype and infection outcome (p = 0.9).
3.2. Biofilm formation capability
All the isolates evaluated were biofilm forming, and according to the quantitative assay for biofilm formation, 52% (26/50), 36% (18/50), and 12% (6/50) of them were strong, moderate, and weak biofilm producers, respectively. The prevalence of strong biofilm producers was 61.53% (16/26) in VAP samples and 38.46% (10/26) in burn samples. In this study, a significant association was observed between being a strong biofilm producer and XDR antimicrobial resistance (p = 0.003). Table 2 shows the distribution of antimicrobial resistance patterns with regarding the of various biofilm‐related genes and different biofilm formation categories. The pattern of biofilm related‐genes among strong and moderate biofilm producers was significantly different compared with weak biofilm producers (Table 2). The distribution of antimicrobial resistance patterns among different biofilm formation categories has been demonstrated in Table 3, indicating a statistically significant association between resistance to gentamicin, imipenem, ticarcillin/clavulanic acid, and ampicillin/sulbactam and strong biofilm formation. Also, resistance to ampicillin/sulbactam was significantly associated with moderate biofilm formation (Table 3).
TABLE 2.
The distribution of antimicrobial resistance patterns and biofilm‐related genes among differenbiofilmproduction phenotypes
| Biofilm formation capacity | Antimicrobial | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MEM | GEN | IMI | CFP | CTZ | CTX | TZP | T/C | AMP/S | |
|
Strong (p value) |
25 (0.26) | 15 (0.04) | 26 (0.007) | 24 (0.18) | 23 (0.91) | 23 (0.37) | 24 (0.18) | 21 (0.02) | 17 (0.000) |
|
Moderate (p value) |
15 (0.09) | 6 (0.25) | 14 (0.09) | 14 (0.20) | 16 (0.88) | 14 (0.36) | 14 (0.20) | 11 (0.58) | 4 (0.03) |
|
Weak (p value) |
6 (0.44) | 1 (0.15) | 4 (0.08) | 5 (0.84) | 5 (0.70) | 5 (0.96) | 5 (0.84) | 1 (0.07) | 0 (0.02) |
| Total | 46 | 22 | 44 | 43 | 44 | 42 | 43 | 33 | 21 |
TABLE 3.
The prevalence of antimicrobial resistant strains with different biofilm formation capacities
| Biofilm formation capacity | Antimicrobial resistance phenotype | Biofilm‐related genes | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| MDR | XDR | cusE | cusD | ompA | ataA | Ptk | ||
| Strong | 14 | 12 | 22 | 26 | 23 | 23 | 24 | 0.003 |
| Moderate | 17 | 1 | 15 | 17 | 14 | 17 | 16 | 0.01 |
| Weak | 6 | 0 | 6 | 6 | 6 | 4 | 5 | 0.31 |
| Non‐biofilm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 37 | 13 | 43 | 49 | 43 | 44 | 45 | |
Abbreviations: AMP/S, ampicillin‐sulbactam; CFP, Cefepime; CTX, ceftriaxone; CTZ, ceftazidime; GEN, gentamicin; IMI, imipenem; MEM, meropenem; T/C, ticarcillin‐clavulanic acid; TZP, piperacillin‐tazobactam.
3.3. Distribution of biofilm‐related genes
The most prevalent gene among all A. baumannii isolates was csuD (98%, 49/50), followed by ptk (90%, 45/50), ataA (88%, 44/50), ompA (86%, 43/50), and csuE (86%, 43/50) (Figure 1). A significantly higher prevalence of ompA was observed in the strains isolated from burn compared with VAP patients (96.2%, 26/27, p = 0.03, OR = 9.175; 95% CI = 1.693–23.80). Twenty six (52%) isolates harbored all the investigated genes. All the isolates harbored more than four biofilm‐related genes. There was a significant difference in the distribution of antimicrobial resistance patterns among strong biofilm producers (p = 0.001).
FIGURE 1.
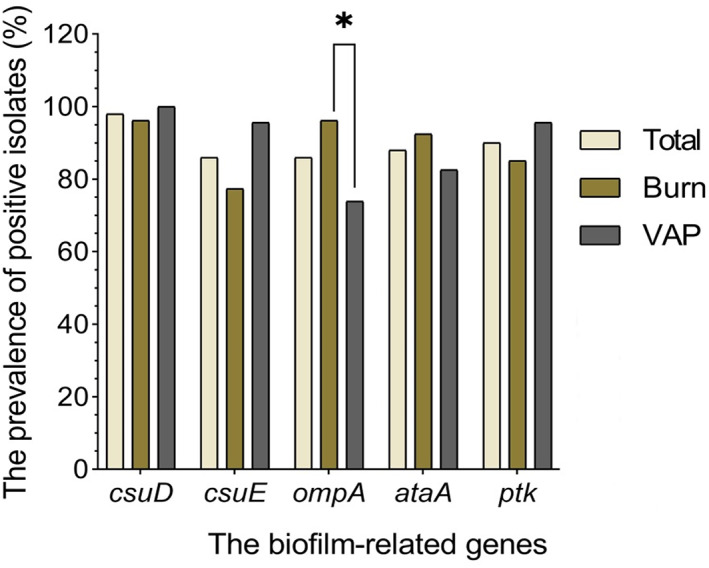
The frequency of biofilm‐related genes in all examined strains isolated from different clinical sources
3.4. Biofilm production capacity among antimicrobial‐resistant strains
The frequency of antimicrobial‐resistant strains was significantly different among isolates with various biofilm production capacities (Figure 2A). Table 3 shows the distribution of antimicrobial resistance patterns among A. baumannii isolates with different capacities for biofilm generation, indicating a higher antimicrobial resistance rate in strong biofilm producers.
FIGURE 2.
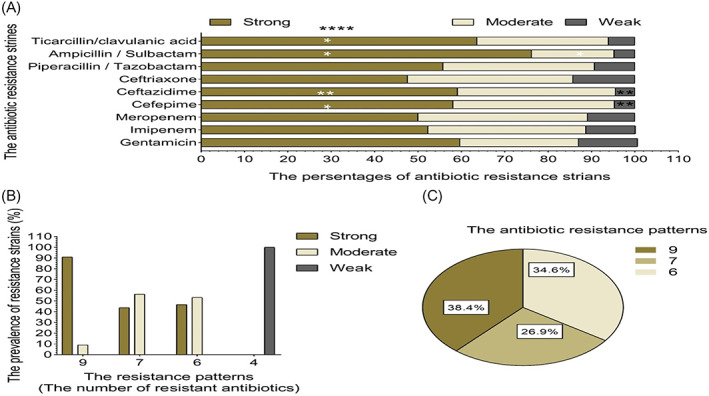
(A) The percentages of antimicrobial resistant strains – resistant strains with different biofilm formation capacities. *p ≤ 0.01; **p ≤ 0.001; ****p ≤ 0.0001. (B) The biofilm formation capacity of isolates with different antimicrobial resistant strains resistance patterns. (C) The distribution of strong biofilm producing isolates among different antimicrobial resistant strains resistance patterns.
Regarding the prevalence of antimicrobial resistance patterns in bacteria with different biofilm formation capacities, strong biofilm producers were more commonly identified with XDR phenotype (Figure 2B). A significant relationship was observed between XDR phenotype and strong biofilm formation (38.46%, 10/26, p = 0.005). Also, the strains harboring all the assessed biofilm‐related genes showed a strong biofilm capacity and a significantly higher prevalence of XDR phenotype (47.62%, 10/21, p = 0.001). However, there was no significant relationship between the distribution of biofilm‐related genes and biofilm formation capacity. The prevalence of XDR A. baumannii with different biofilm formation capacities has been depicted in Figure 2B.
Strong biofilm producers constituted 88.7% (21/26) of the strains harboring all biofilm‐related genes. Our results showed that the presence of all biofilm‐related genes increased the strength of biofilm formation (p < 0.0001).
3.5. Pulsed‐field gel electrophoresis fingerprinting
PFGE was performed for all isolates. The PFGE results showed 6 clusters and 21 different pulsotypes, indicating a remarkable genetic diversity. Among all patients, cluster 1 was the most prevalent (38%) (Figure 3), followed by clusters 2, 5, 4, 3, and 6. The distribution of cluster 3 was restricted to patients with VAP. The lowest prevalence of clusters was in clusters 3 and 4 (0.8%). In clusters 1 and 2, clonality was higher compared to other clusters. In cluster 2, the prevalence of XDR A. baumannii strains with a strong biofilm formation capacity was significantly higher compared to other clusters (p = 0.013, Figure 3). There was no significant relationship between biofilm‐related genes and clusters.
FIGURE 3.
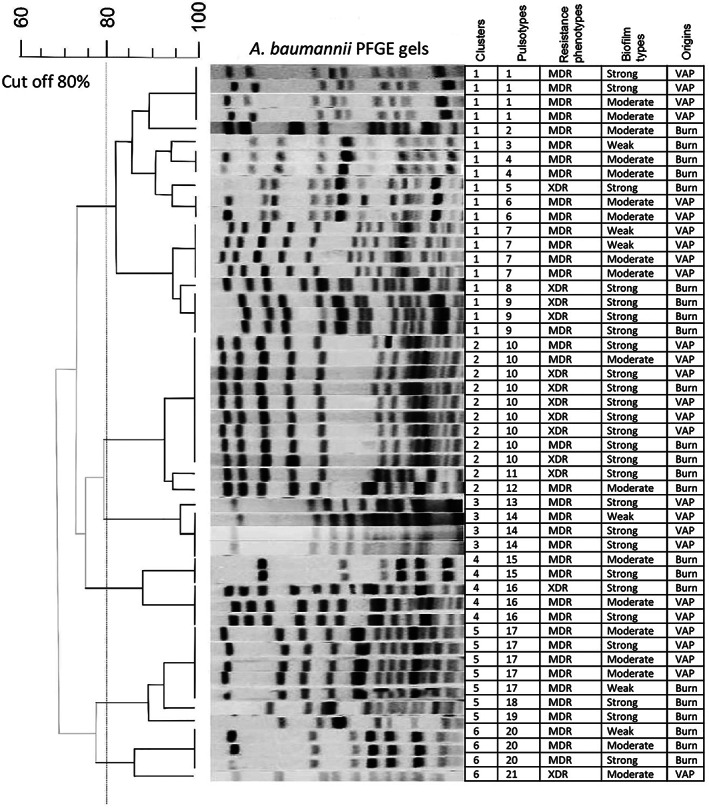
Pulsed field gel electrophoresis was analyzed via Bionumerics using the UPGMA algorithm at the position tolerance of 1.5% and the cut off of 80%.
4. DISCUSSION
MDR A. baumannii poses a great health challenge worldwide, and polymyxin antimicrobials such as colistin, as “salvage” therapy, play an important role against these infections. 24 In this study, all studied isolates were susceptible to colistin and polymyxin‐B, highlighting their importance as rescuing antimicrobials against MDR A. baumannii that has been categorized as an urgent antimicrobial‐resistant infection by the Center for Disease Control (CDC) and World Health Organization (WHO). 25 The development of MDR and especially XDR A. baumannii infections in burn and VAP hospitalized patients poses a great risk factor compromising their improvement and increasing morbidity and mortality in many cases. 26 , 27 A. baumannii infection is particularly common in hospitals and health environments, where its development is mediated by biofilm formation. Within a biofilm niche, bacteria are up to 1000 times more resistant to antimicrobials than the planktonic form. 25
The increase of XDR infections greatly concerns health professionals due to the high rate of antimicrobial therapy failure in the patients admitted to the burn and ICU wards. 26 , 27 Regarding antimicrobial susceptibility, 26% of the assessed isolates exhibited XDR phenotype. In a recent study in Isfahan, Iran, 16.1% of 118 isolates of A. baumannii were XDR. In another study in Zanjan, Iran, Zighami et al. reported that 91% of A. baumannii isolates were XDR, 9 , 28 indicating different frequencies in various geographical regions of the country. In our study, since the sample size was relatively small, non‐biofilm producer isolates were not found, and this limitation should be considered in future studies in the region.
Carbapenem antimicrobials such as meropenem and imipenem have activity against A. baumannii. 29 However, the emergence of carbapenem‐resistant A. baumannii (CRAb) is a serious concern in Iran and other countries. 30 Recently, Beigverdi et al. have reported considerably high resistance rates against meropenem and imipenem among A. baumannii isolates from Iranian patients (83.6% and 81.1%, respectively). 30 In this study, we also observed a high resistance rate of A. baumannii against imipenem and meropenem (88% and 92%, respectively).
As antimicrobial resistance can be acquired by bacteria within a biofilm niche via different molecular mechanisms, such as horizontal gene transfer, plasmid transformation, and DNA uptake, the development of MDR and XDR strains has been noted to associated with biofilm formation on biotic and abiotic surfaces. 22 Accordingly, one of the key findings of this research was that XDR strains tended to form stronger biofilm than MDR strains. The results of this study are quite different from those of Qi et al. who reported that non‐biofilm producer A. baumannii often XDR. 22 On the other hand, Zighami et al. and Khoshnood et al. reported that all MDR and XDR A. baumannii isolates had a strong biofilm formation capacity, highlighting that these strains were often associated with ICU‐related infections. 9 , 31 Shenkutie et al. showed that biofilm formation would induce irreversible resistance in XDR A. baumannii strains. 32
In this study, we showed that the presence of a full set of biofilm‐related genes (ompA, ptk, ataA, csuE, and csuA) predicted strong biofilm formation and variable antimicrobial resistance. An analysis of the biofilm‐related genes of A. baumannii was published by Liu et al. It was found that abaI and csuE were present in 59.8% of the samples and ompA in 100% of the samples. 33 A baumannii isolates from meat of different origins are examined for the presence of biofilm‐related genes by Elbehiry et al. In their study, the prevalence of casE, ompA, bap, and csgA was 72%, 60%, 52.7%, and 25%, respectively. 34
Literature information and our results suggest that ompA‐mediated adhesion contributes significantly to biofilm formation in A. baumanni‐associated nosocomial infections, especially in burn and VAP patients. 35 , 36 In this study, ompA was significantly and more frequently detected in burn than in VAP samples. The ompA protein mediates the interaction between bacteria and epithelial cells. 37 Previous studies have reported a positive relationship between the presence of biofilm‐related genes such as ompA and antimicrobial resistance. 9 , 38
Being the most abundant porin in A. baumannii, the role of OmpA in drug resistance was more prominent in disruption mutants of the gene, which indicated reduced resistance to imipenem, meropenem, nalidixic acid, and chloramphenicol. Also diffusion, studies shows that OmpA possibly couples with efflux pumps and forces out antibacterial compounds from the periplasm. 39 Overproduction of this gene is a risk factor for the mortality rate of nosocomial bacteremia and pneumonia caused by A. baumannii. Besides, the expression level of OmpA measured by qRT‐PCR can be used as a rapid diagnostic index for antibiotic‐resistant A. baumannii. 40 In this study, a strong capacity for biofilm formation significantly correlated with the presence of all the examined biofilm‐related genes, which was in agreement with the results of Amin et al. 36 However, we observed a significant association between being a strong biofilm producer and showing antimicrobial resistance, which was in contrast with the report of Amin et al. who asserted that non‐MDR strains were more capable of generating strong biofilm. 36 This was inconsistent with our observation indicating a higher biofilm formation capacity in XDR than in MDR A. baumannii strains. There are several reports suggesting that XDR bacterial strains form stronger biofilm than antimicrobial ‐sensitive strains, 9 , 41 , 42 which was in parallel to our results showing the higher biofilm formation capability of XDR compared to MDR strains. In contrast with our results; however, a number of studies have reported that sensitive strains form more strong biofilm than MDR bacteria. 22 , 42 , 43
Besides, in our study, harboring all the assessed biofilm‐related genes significantly correlated with XDR phenotype, suggesting a role for biofilm formation in the acquisition of antimicrobial resistance, especially in healthcare facilities and among the bacteria forming biofilm on biotic and abiotic surfaces. 44 Recently, Shenkutie et al. have reported that biofilm formation by A. baumannii during hospital colonization induces transient antimicrobial tolerance in sensitive strains but a more stable resistance in XDR strains. 32 Genetic relatedness is confirmed by several methods and has been addressed by surveillance, subtyping, and epidemiological studies on A. baumannii outbreaks. 45
In this context, Salguero et al. have recently shown in an epidemiological study that matrix‐assisted laser desorption/ionization‐time of flight (MALDI‐TOF) mass spectrometry (MS) and repetitive‐element PCR (Rep‐PCR) are not suitable methods to replace PFGE in the epidemiological evaluation of A. baumannii. 46
In the present study, genome fingerprinting was confirmed by ApaI‐digested PFGE, which is the gold standard for determining genetic relatedness among A. baumannii clinical isolates. 47 The analysis revealed that the distribution of XDR phenotype was significantly high in cluster No. 2; however, no significant correlation was found between clusters and biofilm formation.
Our results were similar to those of Bardbari et al. Three Iranian hospitals were sampled for typing of MDR A. baumannii strains by PFGE to identify the strains they contained. Eight clusters were identified, with two main clusters accounting for 30% and 23% of the sample. In their study, they showed that there was no correlation between biofilm formation and PFGE patterns. 48
The PFGE method was used in a study conducted by Ceparano et al. in which 102 A. baumannii strains isolated from 59 patients were genotyped by this method. Two main patterns were observed as a result of PFGE typing. The results indicated that approximately 40% of the genotyped strains were associated with healthcare‐associated infections, the majority of which were VAP in both infection patterns. 49
In another study conducted in an academic hospital in Turkey, PFGE was used to determine the type of 69 strains of A. baumannii. It has been suggested that different clones may be present in the same hospital departments, while the same clones may be present in different departments. 50
This study investigated the antimicrobial resistance patterns and biofilm formation capacities of MDR A. baumannii strains collected from two Tehran's hospitals, Iran, by the molecular detection of biofilm‐related genes. To the best of our knowledge, there are no previous reports on biofilm formation capacity and the distribution of biofilm‐related genes in A. baumannii, followed by PFGE fingerprinting. Our findings suggested that a strong biofilm formation capacity mediated by biofilm‐related genes might contribute to the acquisition of antimicrobial resistance, especially XDR phenotype, in A. baumannii found in the burn and ICU wards.
5. CONCLUSION
In the present study, the distribution of biofilm‐related genes and antimicrobial resistance patterns were determined in A. baumannii isolated from burn and VAP patients. We also checked for a possible correlation between biofilm formation and antimicrobial resistance patterns. The results demonstrated that XDR phenotype significantly correlated with a strong biofilm formation capacity, and biofilm‐related genes showed a significantly high prevalence in XDR A. baumannii clinical isolates. Our results indicated that the prevalence of antimicrobial resistance correlated with strong biofilm formation, suggesting the transmission of resistance mechanisms among bacterial strains within the biofilm niche. It is suggested to use biofilm disrupting agents to prevent biofilm formation, especially on hospital surfaces, to reduce the extent of the infections caused by MDR and XDR A. baumannii strains, particularly by designing prospective studies.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation were performed by Dr Norkhoda sadeghifard, data collection and analysis were performed by Maryam Mohammadi, Nahid Mahdian, Saeed Khoshnood, Mohammad Hossein Haddadi and Abbas Maleki. The first draft of the manuscript was written by Saeed Khoshnood, Mohammad Hossein Haddadi, Abbas Maleki and Mohsen Heidary and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this study.
Khoshnood S, Sadeghifard N, Mahdian N, et al. Antimicrobial resistance and biofilm formation capacity among Acinetobacter baumannii strains isolated from patients with burns and ventilator‐associated pneumonia. J Clin Lab Anal. 2023;37:e24814. doi: 10.1002/jcla.24814
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Sedaghat A, Khadem‐Rezaiyan M, Ahmadabadi A, et al. Antibacterial resistance pattern of Acinetobacter baumannii in burn patients in Northeast of Iran. Jundishapur. J Microbiol. 2019;12(10):1‐8. [Google Scholar]
- 2. Ramezanalizadeh F, Owlia P, Rasooli I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii . Vaccine. 2020;38(34):5436‐5446. [DOI] [PubMed] [Google Scholar]
- 3. Ashuthosh K, Hegde A, Rao P, Manipura R. Multidrug‐resistant Acinetobacter baumannii–The Modern Menace: a retrospective study in a tertiary hospital in Mangalore. Infect Drug Resist. 2020;13:2181‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babapour E, Haddadi A, Mirnejad R, Angaji S‐A, Amirmozafari N. Biofilm formation in clinical isolates of nosocomial Acinetobacter baumannii and its relationship with multidrug resistance. Asian Pac J Trop Biomed. 2016;6(6):528‐533. [Google Scholar]
- 5. Roy S, Chowdhury G, Mukhopadhyay AK, Dutta S, Basu S. Convergence of biofilm formation and antibiotic resistance in Acinetobacter baumannii infection. Front Med. 2022;9:1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hematian A, Goudarzi H, Ghalavand Z, et al. The relationship between phoH and colistin‐heteroresistant in clinical isolates of Acinetobacter baumannii . Gene Rep. 2021;25:101356. [Google Scholar]
- 7. Magiorakos A‐P, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268‐281. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez T, Stevens ML, Baatyrbek Kyzy A, et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy. 2021;76(1):302‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeighami H, Valadkhani F, Shapouri R, Samadi E, Haghi F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect Dis. 2019;19(1):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kishii K, Hamada M, Aoki K, et al. Differences in biofilm formation and transcription of biofilm‐associated genes among Acinetobacter baumannii clinical strains belonging to the international clone II lineage. J Infect Chemother. 2020;26:693‐698. [DOI] [PubMed] [Google Scholar]
- 11. Weidensdorfer M, Ishikawa M, Hori K, et al. The Acinetobacter trimeric autotransporter adhesin Ata controls key virulence traits of Acinetobacter baumannii . Virulence. 2019;10(1):68‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5(4):e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farajzadeh Sheikh A, Savari M, Abbasi Montazeri E, Khoshnood S. Genotyping and molecular characterization of clinical Acinetobacter baumannii isolates from a single hospital in Southwestern Iran. Pathog Glob Health. 2020;114(5):251‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rafei R, Dabboussi F, Hamze M, et al. Molecular analysis of Acinetobacter baumannii strains isolated in Lebanon using four different typing methods. PLoS One. 2014;9(12):e115969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forbes B, Sahm DF, Weissfelt SA. Bailey and Scott;s Diagnostic Microbiology. Mosby Publication; 2007. [Google Scholar]
- 16. Higgins P, Wisplinghoff H, Krut O, Seifert H. A PCR‐based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin Microbiol Infect. 2007;13(12):1199‐1201. [DOI] [PubMed] [Google Scholar]
- 17. Wayne P., CLSI . Performance Standards for Antimicrobial Susceptibility Testing. CLSI.org; 2020. [Google Scholar]
- 18. Saderi H, Owlia P. Detection of multidrug resistant (MDR) and extremely drug resistant (XDR) P. aeruginosa isolated from patients in Tehran, Iran. Iran J pathol. 2015;10(4):265. [PMC free article] [PubMed] [Google Scholar]
- 19. Abdi‐Ali A, Mohammadi‐Mehr M, Alaei YA. Bactericidal activity of various antibiotics against biofilm‐producing Pseudomonas aeruginosa . Int J Antimicrob Agents. 2006;27(3):196‐200. [DOI] [PubMed] [Google Scholar]
- 20. Mortazavi S, Farshadzadeh Z, Janabadi S, et al. Evaluating the frequency of carbapenem and aminoglycoside resistance genes among clinical isolates of Acinetobacter baumannii from Ahvaz, south‐west Iran. New Microbes New Infect. 2020;38:100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bou G, Cervero G, Dominguez M, Quereda C, Martinez‐Beltran J. PCR‐based DNA fingerprinting (REP‐PCR, AP‐PCR) and pulsed‐field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem‐and meropenem‐resistant Acinetobacter baumannii . Clin Microbiol Infect. 2000;6(12):635‐643. [DOI] [PubMed] [Google Scholar]
- 22. Qi L, Li H, Zhang C, et al. Relationship between antibiotic resistance, biofilm formation, and biofilm‐specific resistance in Acinetobacter baumannii . Front Microbiol. 2016;7:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed‐field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma A, Sharma N, Kumari A, Lee H‐J, Kim T, Tripathi KM. Nano‐carbon based sensors for bacterial detection and discrimination in clinical diagnosis: a junction between material science and biology. Appl Mater Today. 2020;18:100467. [Google Scholar]
- 25. Eze EC, Chenia HY, El Zowalaty ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Res. 2018;11:2277‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Almeida KCF, Calomino MA, Deutsch G, et al. Molecular characterization of multidrug‐resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns. 2017;43(1):137‐143. [DOI] [PubMed] [Google Scholar]
- 27. Osman M, Halimeh B, Rafei R, et al. Investigation of an XDR‐Acinetobacter baumannii ST2 outbreak in an intensive care unit of a Lebanese tertiary care hospital. Future Microbiol. 2020;15:1535‐1542. [DOI] [PubMed] [Google Scholar]
- 28. Monfared AM, Rezaei A, Poursina F, Faghri J. Detection of genes involved in biofilm formation in MDR and XDR Acinetobacter baumannii isolated from human clinical specimens in Isfahan. Iran Arch Clin Infect Dis. 2019;14(2):e85766. [Google Scholar]
- 29. Lenhard JR, Bulitta JB, Connell TD, et al. High‐intensity meropenem combinations with polymyxin B: new strategies to overcome carbapenem resistance in Acinetobacter baumannii . J Antimicrob Chemother. 2016;72(1):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beigverdi R, Sattari‐Maraji A, Emaneini M, Jabalameli F. Status of carbapenem‐resistant Acinetobacter baumannii harboring carbapenemase: first systematic review and meta‐analysis from Iran. Infect Genet Evol. 2019;73:433‐443. [DOI] [PubMed] [Google Scholar]
- 31. Khoshnood S, Savari M, Montazeri EA, Sheikh AF. Survey on genetic diversity, biofilm formation, and detection of colistin resistance genes in clinical isolates of Acinetobacter baumannii . Infect Drug Resist. 2020;13:1547‐1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shenkutie AM, Yao MZ, Siu GK, Wong BKC, Leung PH. Biofilm‐induced antibiotic resistance in clinical Acinetobacter baumannii isolates. Antibiotics. 2020;9(11):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu H, Wu Y‐Q, Chen L‐P, et al. Biofilm‐related genes: analyses in multi‐antibiotic resistant Acinetobacter baumannii isolates from mainland China. Med Science Monit. 2016;22:1801‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elbehiry A, Marzouk E, Moussa IM, et al. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J Biol Sci. 2021;28(1):1158‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Authman SH, Ali FS, Al‐Marjani MF. Biofilm formation in imipenem‐resistant Acinetobacter baumannii from the intensive care unit. J Glob Pharma Technol. 2017;10:404‐411. [Google Scholar]
- 36. Amin M, Navidifar T, Shooshtari FS, et al. Association between biofilm formation, structure, and the expression levels of genes related to biofilm formation and biofilm‐specific resistance of Acinetobacter baumannii strains isolated from burn infection in ahvaz, iran. Infect Drug Res. 2019;12:3867‐3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Payam MA, Rasooli I, Owlia P, Talei D, Astaneh SDA, Nazarian S. Correlation of virulence factors and cell adhesion of clinical isolates of Acinetobacter baumannii . Arc Clin Infect Dis. 2018;13(3):1‐7. [Google Scholar]
- 38. Iyer R, Moussa SH, Durand‐Reville TF, Tommasi R, Miller A. Acinetobacter baumannii OmpA is a selective antibiotic permeant porin. ACS Infect Dis. 2017;4(3):373‐381. [DOI] [PubMed] [Google Scholar]
- 39. Uppalapati SR, Sett A, Pathania R. The outer membrane proteins OmpA, CarO, and OprD of Acinetobacter baumannii confer a two‐pronged defense in facilitating its success as a potent human pathogen. Front Microbiol. 2020;11:589234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nie D, Hu Y, Chen Z, et al. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J Biomed Sci. 2020;27(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gurung J, Khyriem AB, Banik A, Lyngdoh WV, Choudhury B, Bhattacharyya P. Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Ind J Crit Care Med. 2013;17(4):214‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. Antibiograms of multidrug‐resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin‐resistant strains. Clin Infect Dis. 2007;45(5):594‐598. [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez‐Baño J, Marti S, Soto S, et al. Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin Microbiol Infect. 2008;14(3):276‐278. [DOI] [PubMed] [Google Scholar]
- 44. Chaturvedi R, Chandra P, Mittal V. Biofilm formation by Acinetobacter Spp. in association with antibiotic resistance in clinical samples obtained from tertiary care hospital. Res J Pharm Tech. 2019;12(8):3737‐3742. [Google Scholar]
- 45. Gohil N, Panchasara H, Patel S, Singh V. Molecular biology techniques for the identification and genotyping of microorganisms. Microbial Genomics in Sustainable Agroecosystems. Springer; 2019:203‐226. [Google Scholar]
- 46. Garcia‐Salguero C, Culebras E, Alvarez‐Buylla A, Rodriguez‐Avial I, Delgado‐Iribarren A. Usefulness of MALDI‐TOF and REP‐PCR against PFGE for the epidemiological study of Acinetobacter baumannii . Revista Espanola de Quimioterapia. 2021;34(3):207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bansal G, Allen‐McFarlane R, Eribo B. Antibiotic susceptibility, clonality, and molecular characterization of carbapenem‐resistant clinical isolates of Acinetobacter baumannii from Washington DC. Int J Microbiol. 2020;2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohammadi Bardbari A, Mohajeri P, Arabestani MR, et al. Molecular typing of multi‐drug resistant Acinetobacter baumannii isolates from clinical and environmental specimens in three Iranian hospitals by pulsed field gel electrophoresis. BMC Microbiol. 2020;20(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ceparano M, Baccolini V, Migliara G, et al. Acinetobacter baumannii Isolates from COVID‐19 patients in a hospital intensive care unit: molecular typing and risk factors. Microorganisms. 2022;10(4):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gündeşlioğlu ÖÖ, Gökmen TG, Horoz ÖÖ, et al. Molecular epidemiology and antibiotic susceptibility pattern of Acinetobacter baumannii isolated from children in a Turkish university hospital. Turk J Pediatr. 2014;56(4):360‐367. [PubMed] [Google Scholar]
- 51. Abbas JE, Salimizand H, Hassanzadeh S, Ramazanzadeh R. AdeG efflux pump as the main Tigecycline resistance in Acinetobacter baumannii . Gene Rep. 2020;20:100689. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


