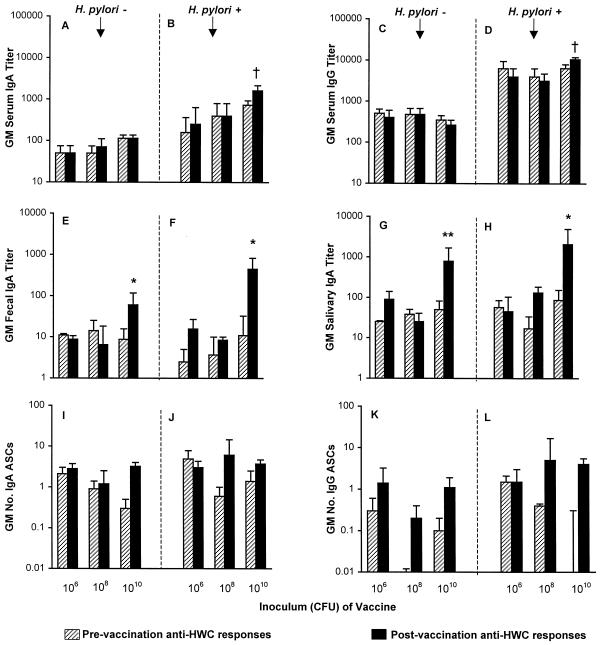
FIG. 1.
Immune responses to HWC antigen according to H. pylori infection status and vaccine dose. Volunteers received an oral dose of either 2.5 × 106, 2.5 × 108, or 2.5 × 1010 inactivated HWC vaccine plus 25 μg of LTR192G adjuvant on days 0, 14, and 28. Responses are expressed as the geometric mean (GM) titer or geometric mean number of ASCs per 106 PBMC ± the back-transformed standard error measured prevaccination and postvaccination. Panels A, C, E, G, I, and K represent volunteers with no evidence of H. pylori infection (there were 3, 4, and 10 recipients of the 106, 108, and 1010 doses of HWC, respectively). Panels B, D, F, H, J, and L represent volunteers with subclinical H. pylori infection at baseline (there were 3, 3, and 8 recipients of the 106, 108, and 1010 doses of HWC, respectively). Comparisons of pre- and postvaccination titers: ∗, P < 0.05; ∗∗, P < 0.01; †, P = 0.06.