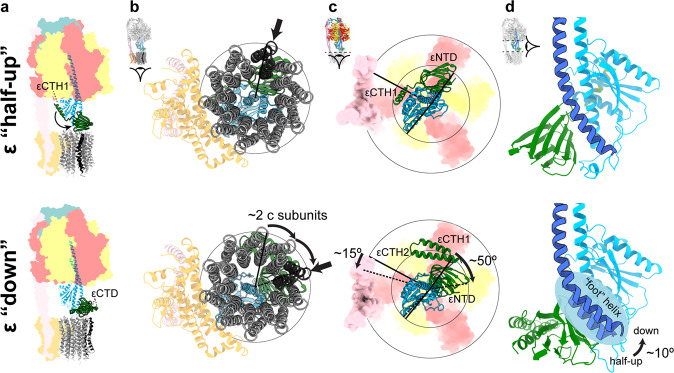
Fig. 4. Structural rearrangements of E. coli F1Fo ATP synthase between the half-up and down conformations.
Comparison of State 2 εCTD half-up conformation (top panels) with State 2 εCTD down conformation (bottom panels). a Overall view describing the εCTD (green) transition and rotation of the c-ring (gray with one c subunit colored black). b When superposed on subunit a (orange), the c-ring rotates two c subunits in the Fo motor (one c subunit colored black with black arrow—relative rotation of c subunits identified using the interaction between εNTD and c subunit; Supplementary Fig. 6). c When superposed on the β barrel crown of the α and β subunits, the εNTD rotates ~50° about the γ subunit and the peripheral stalk flexes ~15°. d When superposed on N-terminus of subunit γ, the foot helix (residues γ39-57) bends and twists to accommodate the movement of the εNTD.