Figure 12.
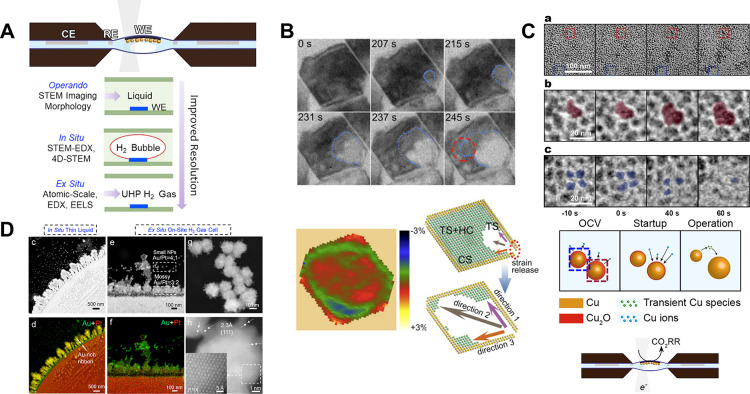
Operando electrochemistry in LC-TEM. (A) Schematics of the SixN LC for operandi LC-TEM with examples of the characterizations and imaging resolution that can be achieved with identical location operando, in situ (with large gas bubble formed), or ex situ. Reproduced from Yang, Y.; Shao, Y.-T.; Lu, X.; Yang, Y.; Ko, H.-Y.; DiStasio, R. A., Jr.; DiSalvo, F. J.; Muller, D. A.; Abruña, H. D. J. Am. Chem. Soc.2022, 144 (34), 15698–15708 (ref (449)). Copyright 2022 American Chemical Society; and from Real-time Monitoring Reveals Dissolution/Redeposition Mechanism in Copper Nanocatalysts during the Initial Stages of the CO2 Reduction Reaction, Vavra, J.; Shen, T.-H.; Stoian, D.; Tileli, V.; Buonsanti, R. Angew. Chem. Int. Ed., Vol. 60, Issue 3 (ref (457)). Copyright 2021 Wiley. (B) Corrosion of Pd@Pt core–shell NPs during the ORR. LC-TEM successive images show the propagation of the dissolution along specific direction corresponding to regions of lower strain (colored map). Reproduced from Chem, Vol. 6 (9), Shi, F.; Gao, W.; Shan, H.; Li, F.; Xiong, Y.; Peng, J.; Xiang, Q.; Chen, W.; Tao, P.; Song, C.; et al., Strain-Induced Corrosion Kinetics at Nanoscale Are Revealed in Liquid: Enabling Control of Corrosion Dynamics of Electrocatalysis, pp. 2257–2271 (ref (453)). Copyright 2020, with permission from Elsevier. (C) Electrochemical Ostwald ripening during eCO2RR at Cu NPs. The NPs in the red square are growing during the reduction step while the smaller ones (blue square) are dissolved into Cu ions. Reproduced from Real-time Monitoring Reveals Dissolution/Redeposition Mechanism in Copper Nanocatalysts during the Initial Stages of the CO2 Reduction Reaction, Vavra, J.; Shen, T.-H.; Stoian, D.; Tileli, V.; Buonsanti, R. Angew. Chem. Int. Ed., Vol. 60, Issue 3 (ref (457)). Copyright 2021 Wiley. (D) Reductive electrodissolution of Au/Pt nanostructures. Operando the formation of a Au ribbon is probed at the Au/Pt interface; higher resolution imaging reveals the detachment and formation of smaller Au NPs from the preferred reductive dissolution of Au. This in situ measurement configuration allows crystallographic analysis of the electrosynthesised Au nanocrystals. Reproduced from Yang, Y.; Shao, Y.-T.; Lu, X.; Yang, Y.; Ko, H.-Y.; DiStasio, R. A., Jr.; DiSalvo, F. J.; Muller, D. A.; Abruña, H. D. J. Am. Chem. Soc.2022, 144 (34), 15698–15708 (ref (449)). Copyright 2022 American Chemical Society.