Abstract
To ensure neuronal survival after severe traumatic brain injury, oxygen supply is essential. Cerebral tissue oxygenation represents the balance between oxygen supply and consumption, largely reflecting the adequacy of cerebral perfusion. Multiple physiological parameters determine the oxygen delivered to the brain, including blood pressure, hemoglobin level, systemic oxygenation, microcirculation and many factors are involved in the delivery of oxygen to its final recipient, through the respiratory chain. Brain tissue hypoxia occurs when the supply of oxygen is not adequate or when for some reasons it cannot be used at the cellular level. The causes of hypoxia are variable and can be analyzed pathophysiologically following “the oxygen route.” The current trend is precision medicine, individualized and therapeutically directed to the pathophysiology of specific brain damage; however, this requires the availability of multimodal monitoring. For this purpose, we developed the acronym “THE MANTLE,” a bundle of therapeutical interventions, which covers and protects the brain, optimizing the components of the oxygen transport system from ambient air to the mitochondria.
Keywords: Brain oxygenation, Cerebral oxygenation monitoring, Brain hypoxia, Cerebral ischemia, Traumatic brain injury
Introduction
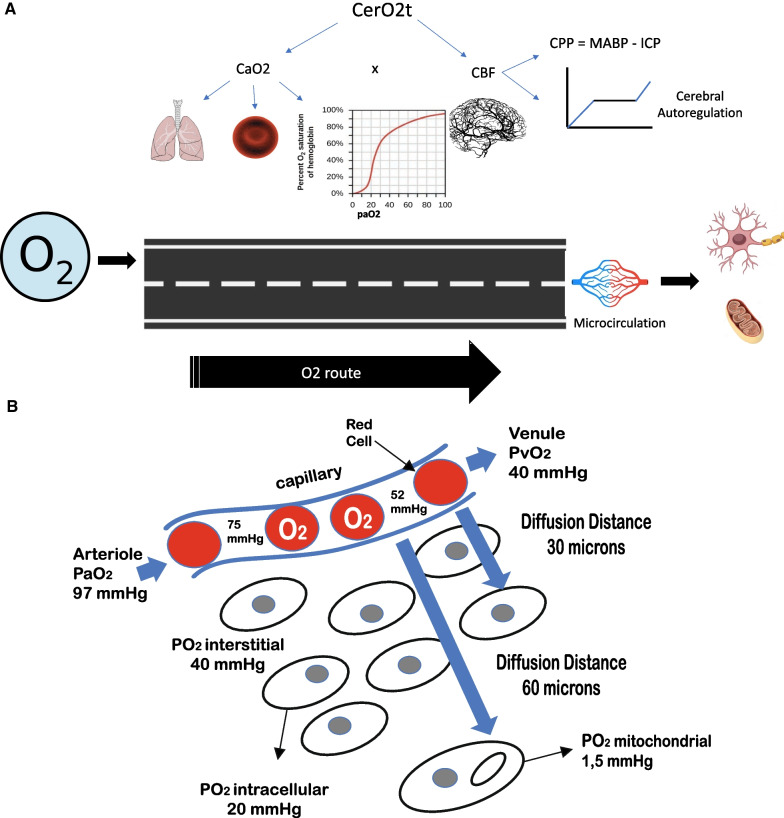
Oxygen (O2) is vital for neuronal survival [1]. Since the brain cannot store O2, it needs its constant supply to maintain its main energy source, which is adenosine triphosphate [1]. Two requirements are essential to ensure the availability of O2 to the brain (DO2): sufficient cerebral blood flow (CBF) and adequate arterial oxygen content (CaO2) [1, 2]. Under physiological conditions, the brain utilizes only 33% of the O2 received, being able to increase extraction when DO2 is compromised in any of its determinants [1, 2]. The achievement of oxygen final metabolism, in the mitochondria, starts from ambient air or a gaseous mixture provided by non-invasive O2 supplemental techniques or mechanical ventilator. This requires a good functioning of the respiratory, cardiovascular (including microcirculation) and hematological systems, all regulated by the state of the internal steady [1, 2]. When DO2 is inadequate or the mitochondria cannot use the supplied O2, ‘‘cerebral tissue hypoxia’’ (CTH) occurs, which constitutes a secondary insult that magnifies the primary brain injury and worsens clinical outcomes, especially in severe traumatic brain injured patients [2]. DO2 is the result of CBF x CaO2, which does not allow the detection of local tissue or microcirculatory abnormalities that limit the local supply of O2 at the tissue level (Fig. 1A and B). CTH is common and prevalent in neurocritical ill patients, and in most cases, it is due to changes in basic physiological parameters [3]. The etiologies of CTH are multiple [4] (Table 1), and can be pathophysiologically approached and investigated following the oxygen route [5] (Fig. 1A and B). However, monitoring of cerebral oxygenation in traumatic brain injured patients is not routinely applied, it has some limitations, and the evidence-based support is not so solid [6, 7]. Even in developed countries, O2 tissue pressure (PtiO2) monitoring rates do not exceed 19% of centers [8]. A recent study suggests that only 8.6% of centers use routinely PtiO2, 1.3% use venous saturation of the jugular bulb (SvjO2) and 1.7% near infrared spectroscopy (NIRS) [9]. Determinants of cerebral oxygenation are multifactorial, and a personalized clinical approach depends on the individual pathophysiological causes. To keep in mind in a practical and simple way the physiological variables involved in the transport and utilization of O2, and help decreasing the occurrence of episodes of cerebral hypoxia, we have created the mnemonic ''THE MANTLE,'' which can be a useful tool at bedside to remind the factors that protect and optimize cerebral oxygenation in severe traumatic brain injured patients (Fig. 2).
Fig. 1.
A Oxygen, O2 route. From atmospheric air or the gaseous mixture supplied by mechanical ventilation, O2 travels following concentration gradients. Cerebral O2 transport (CerO2t) depends on the product of CBF and arterial O2 content (CaO2), determined by the following equation: CaO2 = (Hgb × 1.34 x SaO2) + (PaO2 × 0.003), where: Hgb: concentration in gr/dl; 1.34: number of ml transported by each gram of Hgb; SaO2: arterial O2 saturation; PaO2: arterial pressure of O2. The affinity of oxygen for Hgb is expressed by analyzing the Hgb-oxygen saturation curve. The CBF is mainly determined by the cerebral perfusion pressure (CPP) and the radius of the cerebral resistance vessels (autoregulation curve). B O2 diffusion at cellular level. If the physiological variables interact harmoniously, oxygen reaches the microcirculation at 98 mmHg, then diffuses into the cell through the interstitial space (PO2i = 20–40 mmHg). Inside the cell, the O2 pressure is 1.5 mmHg. The distance that the 02 must travel varies between 20 and 60 microns
Table 1.
Causes and types of cerebral hypoxia
| CBF | PtiO2 | LPR | OEF | Pathophysiology | |
|---|---|---|---|---|---|
| Ischemic | ↓ | ↓ | ↑ | ↑ | Inadequate CBF |
| Low extraction | ≅ | ↓ | ↑ | ≅ | Low arterial partial pressure of oxygen, PaO2 (hypoxemic hypoxia) |
| Low hemoglobin concentration (anemic hypoxia) | |||||
| Low half-saturation pressure P50 (high- affinity hypoxia) | |||||
| Shunt | ↑ | ≅ | ↑ | ↓ | Arteriovenous shunting (microvascular shunt) |
| Diffusion | ≅ | ≅ | ↑ | ↓ | Diffusion barrier (intracellular or interstitial edema) |
| Uncoupling | ≅ | ≅ | ↑ | ↓ | Mitochondrial dysfunction |
| Hypermetabolic | ↑ | ↓ | ↑ | ↑ | Increased demand |
CBF: cerebral blood flow; PtiO2: brain oxygen pressure; LPR: lactate/pyruvate ratio; OEF: oxygen extraction fraction
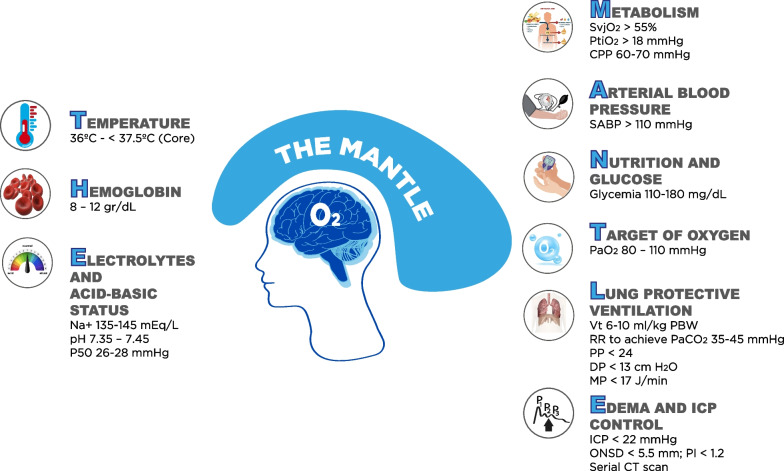
Fig. 2.
The MANTLE mnemonics. Jugular venous saturation of oxygen, SvjO2; brain tissue oxygen pressure, PTiO2; cerebral perfusion pressure, CPP; systolic arterial blood pressure, SABP; tidal volume, Vt; respiratory rate, RR; Plateau pressure, PP; driving pressure, DP; mechanical power, MP; intracranial pressure, ICP; oxygen pressure at half arterial oxygen pressure, p50; optic nerve sheath diameter, ONSD; pulsatility index, PI; Computed Tomography, CT
Temperature: “To avoid hyperthermia is fundamental”
Hyperthermia is highly prevalent in neurocritical patients [10–12]. During the initial phase of brain injury, temperature elevation is commonly attributed to the acute phase response, with inflammatory activation and increased sympathetic activity. Direct damage to the hypothalamic thermoregulatory centers can also cause hyperthermia [10–12]. The brain is warmer than the body, and the difference between brain and central temperature may be up to 2ºC [10, 11]. The presence of fever at ICU admission or during the first hours of evolution constitutes a secondary insult that is associated with the severity of the injury, negatively impacting the final outcomes [10, 11]. Hyperthermia exerts its deleterious effects through various mechanisms: increased levels of excitatory amino acids and free oxygen radicals, inhibition of proteolytic enzymes, rupture of the blood–brain barrier and increased ischemic area in vulnerable regions [10, 11]. Hyperthermia can also yield to cerebral hypoxia due to increased metabolism. Therefore, it is desirable to maintain central temperature levels between 36 and 37ºC [10–12].
Hemoglobin (Hgb): ‘‘To keep and maintain good quality and quantity of transporter is essential’’
Hgb transports more than 95% of the O2 in the blood [1, 13]. Physiologically, each unit decrement in Hgb levels reduces O2 transport capacity (anemic hypoxia), while oxygen delivery does not increase when Hgb values are higher (> 12 gr/dl) [1, 13, 14]. Transfusions do not ensure the correction of cerebral hypoxia due to multiple variables, including amount of blood transfused, and age of donor [3]. The optimal levels of Hgb remain unknown; however, it seems reasonable to reach and maintain Hgb values between 7 and 9 gr/dl [13]. Blood stored for long periods of time decreases its component of 2,3 diphosphoglycerate, which further increases the affinity of Hgb for O2, restricting cell availability [1, 14].
Electrolytes and acid basic status: ‘‘Physiological balance is the cornerstone’’
Homeostasis of the cellular exterior environment is a key factor to ensure physiology of the transport and cession of O2 to the cells. This plays an essential role in avoiding shifts in the Hgb dissociation curve [1, 2, 10]. Both the increase in temperature and carbon dioxide (CO2) and tissue acidosis, product of cellular metabolism, facilitate the transfer of O2 to the tissues by shifting the O2/Hgb dissociation curve to the right. In contrast, hypothermia, hypocapnia, and alkalosis increase the affinity of Hgb for O2 (shift to the left), which makes it more difficult to transfer the necessary O2 to the cell [1]. Acidosis, hypercapnia, and hyperthermia dilate cerebral resistance blood vessels, increasing cerebral blood volume and intracranial pressure, while hypocapnia, by causing vasoconstriction, facilitates cerebral ischemia [1]. In order to ensure that Hgb dissociation curve remains within functional ranges (p50 = 26–28 mmHg), to reduce the risk of cerebral ischemia and intracranial hypertension, the following goals should be achieved: a) pH: 7.35–7.45; b) normocapnia; c) central temperature (T°): 36–37.5 °C [1, 10]. On the other hand, to minimize or treat cerebral edema, it is crucial to maintain a slight hyperosmolar state (serum Na+ 140—150 mEq/L) and to avoid hypotonic fluids [15].
Metabolism: ‘‘If metabolism is accelerated, O2 demands increase’’
Brain metabolism is the main determinant of the rate of cerebral O2 consumption. In some cases of hypoxia, O2 demands exceed supply. For this reason, all those situations that increase the neuronal demand for O2, such as inadequate level of sedation and analgesia (pain, agitation), seizures, fever, sepsis, and paroxysmal sympathetic hyperactivity syndrome, should be investigated and quickly corrected [1, 13, 16]. The cerebral oxygenation goals to be achieved depend on the available resources and the technique employed. Oxygen pressure of the brain parenchyma locally reflects the balance between the supply and consumption of O2 and should be maintained at values above 18 mmHg. The venous oxygen saturation obtained from the jugular bulb (SvjO2), globally represents the O2 that returns to the general circulation after being consumed by brain cells and should be maintained at values > 55%. Both variables depend on adequate CBF, which in turn requires appropriate CPP. When advanced and specialized technology is available, such as microdialysis or a specific software for the continuous evaluation of the autoregulatory phenomenon, it is recommended to maintain the lactate/pyruvate ratio < 25 and pressure reactivity index (Prx) or oxygen reactivity index (Orx) < 0.2. Orx and Prx are the correlation coefficients between CPP and PtiO2 and ICP, respectively. Both parameters are related to cerebral oxygenation, as high ICP reduces oxygen tolerance.
Arterial blood pressure: ‘‘Arterial hypotension is apocalyptic for injured brain’’
One of the main determinants of CBF is CPP, which is the result of mean arterial blood pressure (MABP) minus intracranial pressure (ICP), and depends on the diameter of small cerebral blood vessels (50–150 microns) [13]. These parameters interact giving rise to the cerebral autoregulation curve, an intrinsic phenomenon of resistance in blood vessels that allows, by changing their diameter, to maintain constant CBF [13]. This property is not unlimited, and when autoregulation is impaired, CBF may passively follow the CPP above or below the limits. For years, it has been considered that CBF does not change despite fluctuations in CPP in the range of 50 to 150 mmHg [17]. Recently, physiological studies have challenged this assertion, showing that the phenomenon of cerebral autoregulation is more ''passive'' and the ''plateau'' phase of the autoregulation curve is considerably narrower in brain injured patients [18]. CBF can vary and even become pressure dependent even in physiological situations where blood pressure (BP) varies abruptly such as exercise [18]. Cerebral autoregulation (CAR) is critical to maintain proper brain perfusion and oxygenation. PtiO2 is a surrogate of CBF [19, 20]. CAR can be easily monitored by transcranial Doppler [21] or by an invasive way through MABP manipulation [22] or the utilization of specific software that established the correlation between brain tissue oxygenation and CPP [19, 20]. Orx is an index that evaluates CAR through the relationship between CPP and PtiO2. Orx may vary between − 1 and + 1. When PtiO2 passively follows CPP, autoregulation is compromised, so a positive correlation exists [19, 20]. When autoregulation is intact, PtiO2 is not affected by changes in CPP so there is inverse correlation between both parameters and Orx [19, 20].In turn, MABP depends on different hemodynamic variables such as systemic vascular resistance and cardiac output. In traumatic brain injury, arterial hypotension is one of the factors with the greatest negative impact on the final outcome and can contribute to the development of ischemic hypoxia, and therefore must be urgently prevented and corrected [5, 13].
Recommended blood pressure targets include systolic blood pressure > 100–110 mmHg; normal volemia, diuresis > 30 ml/h, preserved peripheral perfusion, central venous pressure: 6–10 cmH2O [5, 7, 13]. Acceptable CPP levels do not ensure normal brain oxygenation, since there is evidence that brain tissue hypoxia can occur even with normal MABP and ICP values [5]. Furthermore, the concept of personalization of treatment is gaining interest, based on the concept that clinicians should not only consider a common pathophysiological pathway independently from specific brain damage but should adapt the therapeutic management to specific needs [23–26]. For example, one parameter to be considered could be the volume of the contusion [19]. In the presence of a small contusion, the blood brain barrier (BBB) is closed in most of the brain. In this case: 1) the osmolarity is the main driving force for edema formation; 2) autoregulation is efficient (increasing pressure decreases cerebral blood volume); so, 3) the first line treatment may include cerebral fluid drainage, increase in the CPP and osmotherapy [26]. When the contusion volume is greater, the BBB is at least partially open, so: 1) higher osmolarity and pressure may worsen edema; 2) vasogenic edema should be prevented in the contusion area; 3) the first line treatment includes cerebral fluid drainage, deep sedation and perhaps hypothermia [26].
Nutrition and glucose: ‘‘Glucose, essential fuel for the damaged brain’’
Glucose is an essential nutrient and energy substrate to maintain mitochondrial functionality [27]. The injured brain increases its avidity for glucose, and as there is no storage of glucose, no more than 2 min of glucose deprivation are necessary to deplete the scarce cerebral reserves [27]. The consequences of the little availability of glucose to the brain are the main reasons for metabolism compromise. Glycemia levels < 110 mg/dl may cause non-ischemic metabolic crises [28]. In contrast, hyperglycemia > 180 mg/dl causes neurotoxic cascades (inflammation, micro thrombosis, edema) and disturbs the homeostasis of the internal environment (hyperosmolarity, dehydration), compromising the immune status, among other alterations [27]. In addition, neuroglycopenia can contribute to mitochondrial dysfunction (uncoupling hypoxia) [28].
Target of oxygenation: ‘‘Both extremes of systemic oxygenation are deleterious’’
Systemic oxygenation strictly depends on lung function, and the variables that determine gas exchange, especially the ventilation/perfusion ratio and its extremes (dead space and shunt) must be within physiological limits [13]. The increase in dead space causes a decrease in alveolar ventilation, which causes CO2 retention and hypoxemia. On the other hand, the increase in the shunt fraction generates hypoxemia because mixed venous blood perfuses large non-ventilated areas, not allowing arterial blood to become enriched in O2. Markers of this type of hypoxemic hypoxia are the decrease in arterial oxygen pressure (PaO2) and arterial oxygen saturation (SaO2), bearing in mind that PaO2 represents dissolved O2, that affects only 3–4% of the total of oxygen transport capacity [1].
In this context, a common and rational practice would be to increase the fraction of inspired O2 (FiO2); however, this measure does not solve the underlying problem without an exhaustive analysis of the situation, since even with supranormal levels of PaO2 (normobaric hyperoxia) hidden cerebral hypoxia can still occur; on the other side, recent evidence suggested that even hyperoxia can be harmful [29].
If these variables are compromised, measures must be taken to achieve PaO2 80–120 mmHg, and SaO2 > 95% [30].
Lung protective ventilation: ‘‘Protecting the lungs protects the brain’’
The concept of lung protective ventilation is challenging in brain injured patients. In fact, the combination of low tidal volume (to keep low plateau pressure and driving pressure) with high intrathoracic pressures and reduced venous outflow induced by positive end expiratory pressure (PEEP) might favor an increase in carbon dioxide value. For these reasons, traditionally, this population of patients has been excluded from the major trials investigating protective strategies in the general ICU population, and no strong evidence is available on this topic [30, 31].
However, over the last years, the concept of lung protective ventilation is gaining interest even in brain injured patients, as it can reduce pulmonary complications and therefore be associated with improved outcomes [30, 31].
Optimizing mechanical ventilator strategies means optimizing lung function and systemic and cerebral oxygenation, but at the same time reducing the risk of ischemic hypoxia secondary to vasoconstriction (hypocapnia) and intracranial hypertension for vasodilatation (hypercapnia) [3, 5, 7].
According to available evidence, it seems prudent to start lung protective ventilation with a controlled mode, tidal volumes between 6 and 8 ml/ kg, minimum respiratory rates to ensure levels of PaCO2 between 35 and 45 mmHg, and FiO2 and PEEP necessary to achieve systemic oxygenation targets as we mentioned above [30, 31]. To prevent mechanical ventilation induced lung injury (barotrauma, biotrauma, volutrauma) plateau pressure should be kept < 2 cmH2O, driving pressure < 13 cm H2O [30, 31] and mechanical power below 17 J/min [32]. It is recommended not to use routinely hyperventilation and to maintain PaCO2 levels between 35 and 45 mmHg [7]. Lower targets can be used as strategies to control intracranial hypertension [22]. In life threatening situations, such as herniation syndromes, plateau waves type A or intracranial hypertension secondary to hyperemia, moderate and controlled hyperventilation can be used [33, 34].
Edema and ICP control: ‘‘Brain swollen, brain on the ledge’’
Cerebral edema contributes to the development of cerebral tissue hypoxia through two mechanisms. On one hand, it can cause ischemic hypoxia by increased ICP with consequent decrease in CPP; on the other hand, it contributes to the development of hypoxia by reducing diffusion of O2 to the cells [35, 36], Fig. 2. Achievement of appropriate levels of sodium is essential to minimize cerebral edema [15]. Also, the application of established intracranial hypertension management protocol is recommended to treat intracranial hypertension [22, 37–40]. The recommended main targets to be achieved should be the following: a) ICP < 22 mmHg; b) CPP: 55–70 mmHg; c) optic nerve sheath diameter (ONSD) < 5.8 mm; d) pulsatility index (PI) < 1.2; and e) Cerebral CT scan without edema signs.
Conclusion
CTH is not uncommon in severe traumatic brain injury and independently predicts poor outcomes. Knowledge of the physiology and kinetics of O2 and of the various causes of hypoxia, together with clinical reasoning and personalized treatment can help to minimize the incidence of CTH and its direct and dangerous consequences even without advanced and specific neuromonitoring.
Acknowledgements
Not applicable
Abbreviations
- O2
Oxygen
- DO2
Oxygen availability
- CBF
Cerebral blood flow
- CTH
Cerebral tissue hypoxia
- PtiO2
Brain oxygen tissue pressure
- SvjO2
Jugular bulb venous oxygen saturation
- NIRS
Near infrared spectroscopy
- Hgb
Hemoglobin
- ºC
Centigrade degrees
- ICP
Intracranial pressure
- CPP
Cerebral perfusion pressure
- MABP
Mean arterial blood pressure
- BP
Blood pressure
- CAR
Cerebral autoregulation
- TBI
Traumatic brain injury
- paO2
Arterial partial pressure of oxygen
- SaO2
Arterial oxygen saturation
- FiO2
Inspired oxygen fraction
- p50
Oxygen pressure at half arterial oxygen saturation
- paCO2
Arterial carbon dioxide pressure
- PEEP
Positive end expiratory pressure
- OSND
Optic sheath nerve diameter
- PI
Pulsatility index
- CT
Computed tomography
Author contributions
DAG gave original idea, wrote the first draft and to perform Table 1 and Fig. 1A and B and was involved in discussion and edition of final version. CR, FMC, JIS, RB and PP were involved in discussion, critical analysis and edition of the manuscript. RB and CR are involved in drafting Fig. 2. All authors read and approved the final manuscript.
Funding
The authors of this manuscript declare that they have not received funds or fees for writing it.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel Agustin Godoy, Email: dagodoytorres@yahoo.com.ar.
Francisco Murillo-Cabezas, Email: franciscomurillocabezas@gmail.com.
Jose Ignacio Suarez, Email: jsuarez5@jhmi.edu.
Rafael Badenes, Email: rafaelbadenes@gmail.com.
Paolo Pelosi, Email: ppelosi@hotmail.com.
Chiara Robba, Email: kiarobba@gmail.com.
References
- 1.Zauner A, Daugherty WP, Bullock MR, et al. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002;51:289–301. [PubMed] [Google Scholar]
- 2.Marín-Caballos AJ, Murillo-Cabezas F, Domínguez-Roldan JM, Leal-Noval SR, Rincón-Ferrari MD, Muñoz-Sánchez MÁ. Monitorización de la presión tisular de oxígeno (PtiO2) en la hipoxia cerebral: aproximación diagnóstica y terapéutica. Med Intensiva. 2008;32(2):81–90. doi: 10.1016/S0210-5691(08)70912-4. [DOI] [PubMed] [Google Scholar]
- 3.Oddo M, Levine JM, Mackenzie L, Frangos S, Feihl F, Kasner SE, Katsnelson M, Pukenas B, Macmurtrie E, Maloney-Wilensky E, Kofke WA, LeRoux PD. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery. 2011;69(5):1037–45. doi: 10.1227/NEU.0b013e3182287ca7. [DOI] [PubMed] [Google Scholar]
- 4.Siggarard-Andersen O, Gothgen IH, Fogh-Andersen N, Larsen LH. Oxygen status of arterial and mixed venous blood. Crit Care Med. 1995;23:1284–1293. doi: 10.1097/00003246-199507000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Godoy DA, Badenes R, Murillo-Cabezas F. Ten physiological commandments for severe head injury. Rev Esp Anestesiol Reanim (Engl Ed) 2021;68(5):280–292. doi: 10.1016/j.redar.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Oddo M, Bösel J; Participants in the International Multidisciplinary Consensus Conference on Multimodality. Monitoring Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care. 2014; 21 Suppl 2: S103–20. [DOI] [PubMed]
- 7.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurg. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 8.Sivakumar S, Taccone FS, Rehman M, Hinson H, Naval N, Lazaridis C. Hemodynamic and neuro-monitoring for neurocritically ill patients: an international survey of intensivists. J Crit Care. 2017;39:40–47. doi: 10.1016/j.jcrc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Llompart-Pou JA, Barea-Mendoza JA, Sánchez-Casado M, González-Robledo J, Mayor-García DM, Montserrat-Ortiz N, Enríquez-Giraudo P, Cordero-Lorenzana ML, Chico-Fernández M. En representación del Grupo de Trabajo de Neurointensivismo y Trauma de la SEMICYUC. Neuromonitoring in the severe traumatic brain injury. Spanish Trauma ICU Registry (RETRAUCI) Neurocirugia (Astur). 2019 doi: 10.1016/j.neucir.2019.05.005. [DOI] [Google Scholar]
- 10.Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37(Suppl):S250–S257. doi: 10.1097/CCM.0b013e3181aa5e8d. [DOI] [PubMed] [Google Scholar]
- 11.Bao L, Chen D, Ding L, Ling W, Xu F. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS ONE. 2014;9:e90956. doi: 10.1371/journal.pone.0090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puccio AM, Fischer MR, Jankowitz BT, Yonas H, Darby JM, Okonkwo DO. Induced normothermia attenuates intracranial hypertension and reduces fever burden after severe traumatic brain injury. Neurocrit Care. 2009;11:82–87. doi: 10.1007/s12028-009-9213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godoy DA, Lubillo S, Rabinstein AA. Pathophysiology and management of intracranial hypertension and tissular brain hypoxia after severe traumatic brain injury: an integrative approach. Neurosurg Clin N Am. 2018;29:195–212. doi: 10.1016/j.nec.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lelubre C, Bouzat P, et al. Anemia management after acute brain injury. Crit Care. 2016;20:152. doi: 10.1186/s13054-016-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterns RH. Disorders of plasma sodium–causes, consequences, and correction. N Engl J Med. 2015;372:55–65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 16.Taran S, Pelosi P, Robba C. Optimizing oxygen delivery to the injured brain. Curr Opin Crit Care. 2022;28:145–156. doi: 10.1097/MCC.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 17.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1951;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 18.Brassard, P., Labrecque, L., Smirl, J. D., Hannah, M. T., Ryan, G. C., Lucas, S. J. E. Losing the dogmatic view of cerebral autoregulation. Physiological Reports, 2012; 10.14814/phy2.14982. [DOI] [PMC free article] [PubMed]
- 19.Jaeger M, Soehle M, Schuhmann MU, Winkler D, Meixensberger J. Correlation of continuously monitored regional cerebral blood flow and brain tissue oxygen. Acta Neurochir (Wien) 2005;147:51–56. doi: 10.1007/s00701-004-0408-z. [DOI] [PubMed] [Google Scholar]
- 20.Valadka AB, Hlatky R, Furuya Y, Robertson C. Brain tissue PO2: correlation with cerebral blood flow. Acta Neurochir Suppl (Wien) 2002;81:299–330. doi: 10.1007/978-3-7091-6738-0_76. [DOI] [PubMed] [Google Scholar]
- 21.Smielewski P, Czosnyka M, Kirkpatrick P, Pickard JD. Evaluation of the transient hyperemic response test in head-injured patients. J Neurosurg. 1997;86:773–778. doi: 10.3171/jns.1997.86.5.0773. [DOI] [PubMed] [Google Scholar]
- 22.Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intensive Care Med. 2020;46:919–929. doi: 10.1007/s00134-019-05900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robba C, Taccone FS, Citerio G. Monitoring cerebral oxygenation in acute brain-injured patients. Intensive Care Med. 2022 doi: 10.1007/s00134-022-06788-w. [DOI] [PubMed] [Google Scholar]
- 24.Chesnut RM, Videtta W. Situational intracranial pressure management: an argument against a fixed treatment threshold. Crit Care Med. 2020;48:1214–1216. doi: 10.1097/CCM.0000000000004395. [DOI] [PubMed] [Google Scholar]
- 25.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 26.Lescot T, Degos V, Zouaoui A, Préteux F, Coriat P, Puybasset L. Opposed effects of hypertonic saline on contusions and noncontused brain tissue in patients with severe traumatic brain injury. Crit Care Med. 2006;34:3029–3033. doi: 10.1097/01.CCM.0000243797.42346.64. [DOI] [PubMed] [Google Scholar]
- 27.Godoy DA, Behrouz R, Di Napoli M. Glucose control in acute brain injury: does it matter? Curr Opin Crit Care. 2016;22:120–127. doi: 10.1097/MCC.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 28.Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Taccone FS, He X2. Harmful effects of hyperoxia in postcardiac arrest, sepsis, traumatic brain injury, or stroke: the importance of individualized oxygen therapy in critically Ill patients. Can Respir J. 2017; 2017: 2834956. [DOI] [PMC free article] [PubMed]
- 30.Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European society of intensive care medicine consensus. Intensive Care Med. 2020;46:2397–2410. doi: 10.1007/s00134-020-06283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tejerina EE, Pelosi P, Robba C, Peñuelas O, Muriel A, Barrios D, Frutos-Vivar F, et al; VENTILA Group. Evolution Over Time of Ventilatory Management and Outcome of Patients With Neurologic Disease. Crit Care Med. 2021; 49: 1095–1106. [DOI] [PubMed]
- 32.Robba C, Badenes R, Battaglini D, et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: a preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) trial. Intensive Care Med. 2022 doi: 10.1007/s00134-022-06756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Della Torre V, Badenes R, Corradi F, Racca F, Lavinio A, Matta B, Bilotta F, Robba C. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis. 2017;9(12):5368–5381. doi: 10.21037/jtd.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picetti E, Pelosi P, Taccone FS, Citerio G, Mancebo J, Robba C; on the behalf of the ESICM NIC/ARF sections. VENTILatOry strategies in patients with severe traumatic brain injury: the VENTILO Survey of the European Society of Intensive Care Medicine (ESICM). Crit Care. 2020; 24: 158. [DOI] [PMC free article] [PubMed]
- 35.Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, Cazati DC, Cordioli RL, Correa TD, Pollard TJ, Schettino GPP, Timenetsky KT, Celi LA, Pelosi P, Gama de Abreu M, Schultz MJ; PROVE Network Investigators. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018; 44: 1914–1922. [DOI] [PubMed]
- 36.Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation therapy for control of posttraumatic intracranial hypertension. Front Neurol. 2017;8:250. doi: 10.3389/fneur.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gouvea Bogossian E, Peluso L, Creteur J, Taccone FS. Hyperventilation in adult TBI patients: how to approach it? Front Neurol. 2021;11:580859. doi: 10.3389/fneur.2020.580859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA, Aigbirhio F, Skepper JN, Minhas PS, Hutchinson PJ, Carpenter TA, Clark JC, Pickard JD. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32:1384–1390. doi: 10.1097/01.CCM.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- 39.Veenith TV, Carter EL, Geeraerts T, Grossac J, Newcombe VF, Outtrim J, Gee GS, Lupson V, Smith R, Aigbirhio FI, Fryer TD, Hong YT, Menon DK, Coles JP. Pathophysiologic Mechanisms of Cerebral Ischemia and Diffusion Hypoxia in Traumatic Brain Injury. JAMA Neurol. 2016;73:542–550. doi: 10.1001/jamaneurol.2016.0091. [DOI] [PubMed] [Google Scholar]
- 40.Godoy DA, Rabinstein AA. How to manage traumatic brain injury without invasive monitoring? Curr Opin Crit Care. 2022;1(28):111–122. doi: 10.1097/MCC.0000000000000914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.