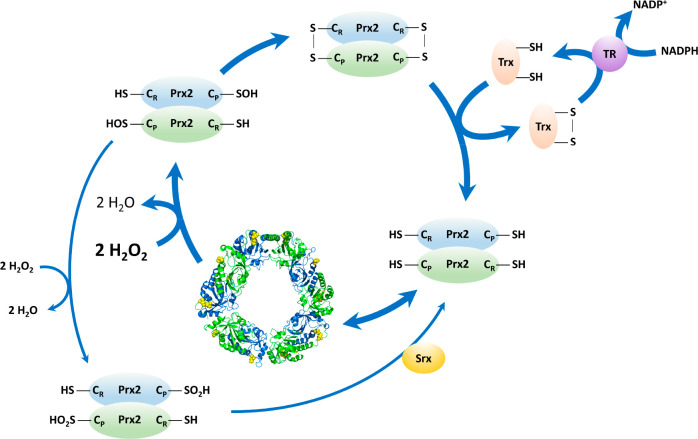
Figure 2.
Peroxiredoxin activity. The reduced Cys52 in Prx2 (CPSH) is oxidized by H2O2 and other oxidants to sulfenic acid (CP-SOH). This CPSOH reacts with the CRSH, forming an intermolecular disulfide bridge. The disulfide-oxidized Prx2 is predominantly a dimer and is reduced by Trx, TR, and NADPH. The oxidized CPSOH can alternatively react with a second oxidant molecule to yield the hyperoxidized sulfinic acid (CPSO2). The latter can either be repaired to the active enzyme by sulfiredoxin (Srx) or form stacked decamer high molecular weight structures. The structure of decameric Prx2 (5IJT) shows reactive cysteine residues in yellow, and each dimer is shown in green and blue, as sides of the pentagon.