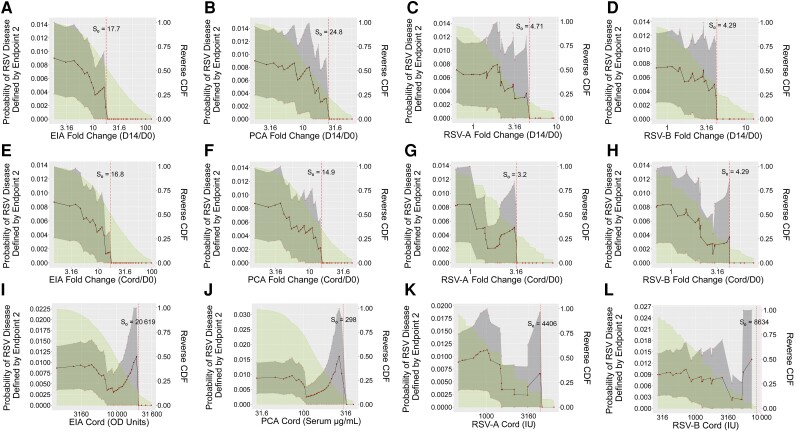
Figure 4.
Risk of respiratory syncytial virus (RSV) disease (defined by endpoint 2) in vaccine arm subgroups defined by antibody marker exceeding thresholds with antibody marker defined by fold-change (day 14/day 0 [D0]) in enzyme immunoassay (EIA) (A), palivizumab-competitive antibody (PCA) (B), RSV-A (C), or RSV-B (D); fold-change (infant cord blood [Cord]/D0) in EIA (E), PCA (F), RSV-A (G), or RSV-A (H); or Cord levels of EIA (I), PCA (J), RSV-A (K), or RSV-B (L), with adjustment for covariates. The Cord/D0 fold-change analyses were post hoc. The gray-shaded region indicates pointwise 95% confidence intervals and the green shaded region is the area under the reverse cumulative distribution function. The vertical dashed red line marks the threshold of estimated zero risk. Endpoint 2 was defined as RSV-associated lower respiratory tract infection with severe hypoxemia (14 vaccine endpoints). Abbreviations: CDF, cumulative distribution function; Cord, infant cord blood; D0, day 0; D14, day 14; EIA, enzyme immunoassay; OD, optical density; PCA, palivizumab-competitive antibody; RSV-A, respiratory syncytial virus subtype A; RSV-B, respiratory syncytial virus subtype B.