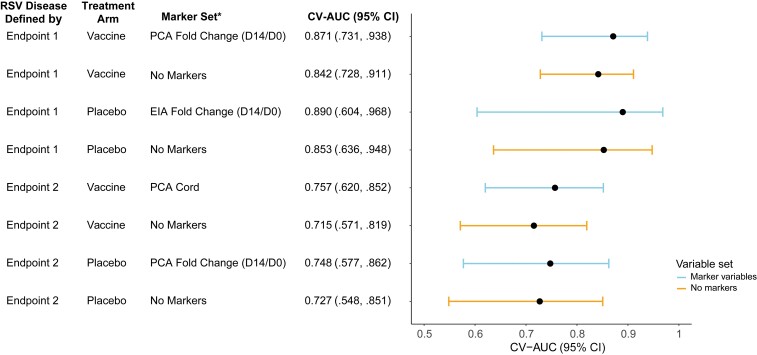
Figure 5.
Performance by treatment arm of SuperLearner for predicting respiratory syncytial virus (RSV) disease case/control status defined by endpoint 1 or by endpoint 2. Cases were defined as infants in the correlates analysis cohort with an RSV illness defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Controls were defined as infants who did not experience RSV disease defined by endpoint 1 or endpoint 2 (as appropriate) through 90 days of age in the expanded data set. Endpoint 1 was defined as medically significant RSV-associated lower respiratory tract infection (LRTI), RSV-associated LRTI with hospitalization, or RSV-associated LRTI with severe hypoxemia (52 vaccine and 51 placebo endpoints). Endpoint 2 was defined as RSV-associated LRTI with severe hypoxemia (14 vaccine and 27 placebo endpoints). *All models include maternal baseline covariates. Abbreviations: CI, confidence interval; Cord, infant cord blood; CV-AUC, cross-validated area under the receiver operating characteristic curve; D0, day 0; D14, day 14; EIA, enzyme immunoassay; PCA, palivizumab-competitive antibody; RSV, respiratory syncytial virus.