Figure 1: Risk stratification for patients with acute lymphoblastic leukaemia (ALL) according to the criteria set by the ALL IC-BFM 2002 trial58,78.
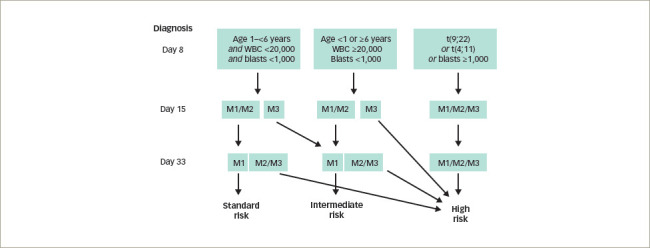
ALL IC-BFM 2002: Acute Lymphoblastic Leukemia Intercontinental Berlin-Frankfurt-Münster 2002. Standard risk: prednisone good response (<1×109/L blasts in peripheral blood on day 8 after 7 days of prednisone and one dose of intrathecal methotrexate on day 1), age 1–6 years, initial WBC <20×109/L, and M1 (<5% blasts) or M2 (5–25% blasts) marrow on day 15, and M1 marrow on day 33.
Intermediate risk: prednisone good response, age <1 year or ≥6 years, and/or WBC ≥20×109/L and M1 or M2 marrow on day 15 and M1 marrow on day 33, or standard-risk criteria but M3 (≥25% blasts) marrow on day 15 and M1 marrow on day 33.
High risk: prednisone poor response (≥1×109/L blasts in peripheral blood on day 8 after 7 days of prednisone and one dose of intrathecal methotrexate on day 1), or intermediate risk and M3 marrow on day 15, or M2 or M3 marrow on day 33, or existence of chromosomal rearrangements t(9;22) (BCR-ABL) or t(4;11) (MLL-AF4).
M1 = <5% bone marrow blasts; M2 = 5–25% bone marrow blasts; M3 = >25% bone marrow blasts; WBC = white blood cells.
Modified with permission from: Grinspon et al.59, copyright 2019 The Authors. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
