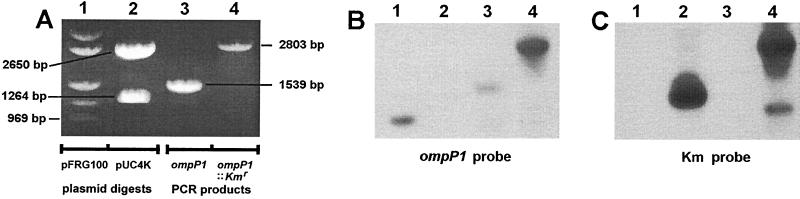
FIG. 4.
Characterization of the putative P1-deficient isogenic mutant of NTHI BCH-3 by Southern blot analysis. (A) Agarose gel electrophoretic separation of digests and PCR products, stained with ethidium bromide. Lane 1, HindIII/PstI double digest of the ompP1-carrying vector plasmid pFRG100; the bottom band is the predicted 969-bp ompP1 fragment (see Fig. 2); lane 2, BamHI digest of pUC4K; the bottom band is the predicted 1,264-bp Kmr cassette, and the upper band is the predicted 2,650-bp backbone of pUC4K (see Fig. 2); lane 3, PCR amplification of the ompP1 gene (1,371 bp) plus flanks (109 bp upstream and 59 bp downstream) from wt BCH-3 (13; unpublished data) using flanking primers P1-5 and P1-6 (see Materials and Methods) to generate a 1,539-bp product; lane 4, PCR amplification of ompP1 from the putative, insertionally inactivated ompP1 BCH-3 Kmr transformant using flanking primers P1-5 and P1-6 (Materials and Methods); when this product is compared with the ompP1 PCR product in the adjacent lane 3, the size increase is found to correlate with that predicted for insertion of the 1,264-bp Kmr cassette as indicated in Fig. 2. (B) Southern blot transfer of the panel A gel electrophoretic separation to a Zeta Probe nylon membrane (Bio-Rad), followed by hybridization with a 32P-labeled (2) ompP1 969-bp fragment probe (the bottom HindIII/PstI fragment shown in panel A, lane 1, as isolated from an equivalent digest and electrophoretic separation). (C) Duplicate electrophoretic separation of the identical digests and PCR products in panel A, transferred by Southern blotting to a Zeta Probe nylon membrane and hybridized with a 32P-labeled (2) Kmr cassette probe (the smaller, 1,264-bp BamHI fragment shown in panel A, lane 2, as isolated from an equivalent digest and electrophoretic separation). The lower band in lane 4 of panel C is likely a PCR breakdown product.