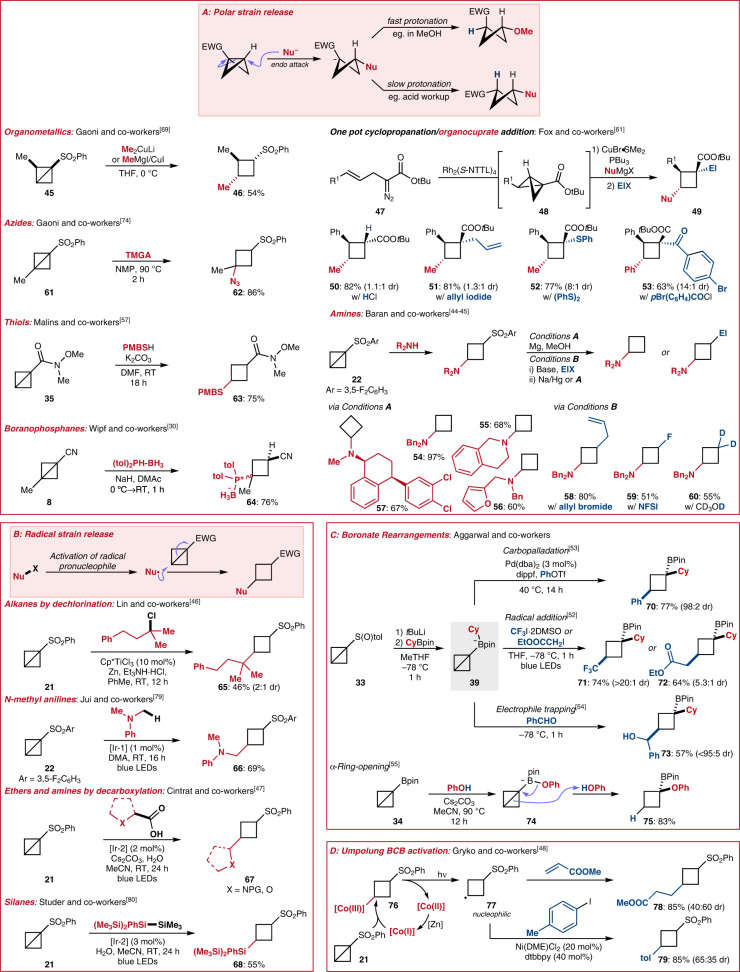
Fig. 3. Strain-release-driven ring-opening of BCBs to give substituted cyclobutanes.
A Polar strain-release reactions with polar nucleophiles. B Radical strain-release reactions with radical nucleophiles. C Strain-release reactions driven by boronate rearrangement chemistry. Both polar and radical electrophiles can be used in these reactions. D Umpolung BCB activation with Co(I) complexes. Formal nucleophilic attack of the Co(I)-complex into the bicyclobutane gives a Co(III)-intermediate from which a cyclobutyl radical may be photochemically generated. Nu nucleophile, El electrophile, EWG electron-withdrawing group, TMGA tetramethylguanidinium azide, PMB para-methoxy benzyl, tol tolyl, NTTL N-1,8-naphthaloyl-(S)-tert-leucine, NFSI N-fluorobenzenesulfonimide, [Ir-1] [Ir{dF(CF3)ppy}2(dtbbpy)]PF6, [Ir-2] [Ir(dF(CF3)ppy)2(5,5’-d(CF3)bpy)]PF6, dba dibenzylideneacetone, dippf 1,1′-bis(diisopropylphosphino)ferrocene, Tf trifluoromethylsulfonyl, pin pinacol, DME dimethoxyethane, dtbbpy 4,4′-di-tert-butyl-2,2′-dipyridyl; Cp* 1,2,3,4,5-pentamethylcyclopentadienyl.