Abstract
The treatment landscape of advanced hepatocellular carcinoma (HCC) has broadened with immune checkpoint inhibitors (ICIs) setting a novel standard of care. With the increased number of therapies either in first or in further line, disentangling the possible treatment sequences has become much more complex. Yet, all the second-line therapies have been evaluated after sorafenib. After ICIs, offering multikinase inhibitors is a widespread approach, either shifting forward sorafenib or lenvatinib, or choosing among regorafenib or cabozantinib, already approved in the refractory setting. Under specific circumstances, ICIs could be maintained beyond disease progression in patients with proven clinical benefit, as supported by some data emerging from phase III clinical trials with immunotherapy in HCC. Rechallenge with ICIs is an additional attractive alternative, although requiring careful and individual evaluation as efficacy and safety of such a strategy have not been yet clarified. Still, a considerable number of patients displays primary resistance to ICIs and might benefit from antiangiogenics either alone or in addition to ICIs instead. Hopefully, the ongoing clinical trials will enlighten regarding the most effective treatment pathways. The identification of predictive correlates of response to immunotherapy will help treatment allocation at each stage, thus representing an urgent matter to address in HCC research. With programmed death ligand 1 expression, tumor mutational burden, and microsatellite status being inadequate biomarkers in HCC, patient characteristics, drug safety profile, and regulatory approval remain key elements to acknowledge in routine practice. Despite the tissue remaining a preferred source, biomarkers discovery could take advantage of liquid biopsy to overcome the matter of tissue availability and track tumor changes. Lastly, tumor genetic phenotypes, tumor microenvironment features, gut microbiome, and markers of immune response and systemic inflammation are all potential emergent predictors of response to ICIs, pending validation in the clinical setting.
Keywords: hepatocellular carcinoma, immunotherapy, immune checkpoint inhibitors, liver cancer, multikinase inhibitors, rechallenge, sequencing, systemic therapy, targeted therapy, tyrosine kinase inhibitors
Introduction
Liver cancer is one of the most common cancers worldwide and remains a global health challenge.1 Although Africa and Asia are the most affected countries, its incidence is rising in Western countries as well and it is predicted that there will be over one million individuals affected annually by liver cancer by 2025.2 Hepatocellular carcinoma (HCC) – which accounts for around 90% of the cases of primary liver cancer – generally arises on a cirrhotic liver as a result of hepatitis B virus or hepatitis C virus infection, excessive alcohol consumption, metabolic syndrome, and exposure to toxic agents such as aflatoxin.1 Its management is informed by the Barcelona Clinic Liver Cancer staging system, the most widely used system for the disease, which endorses the use of systemic therapy for patients diagnosed at an advanced stage and fit for treatment.3
The therapeutic landscape of advanced HCC has changed significantly over recent years. Multityrosine kinase inhibitors (MKIs) were the first class of agents showing an overall survival (OS) benefit with sorafenib being approved in 2007 following the positive results of two randomized phase III trials.4,5 A decade later, lenvatinib was found to be non-inferior compared to sorafenib in the first-line setting.6 In the refractory setting, regorafenib in a sorafenib-tolerant population and cabozantinib showed to be an effective second-line option when compared to placebo.7,8 Of note, cabozantinib is also approved as a third-line option as 27% of the patients in the CELESTIAL trial received it in this setting, although the trial was not powered to demonstrate a survival difference according to the line of treatment.8 In addition, ramucirumab, a recombinant immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that binds to vascular endothelial growth factor receptor 2 (VEGFR-2), demonstrated improved OS compared to placebo in patients with baseline alpha-fetoprotein (AFP) levels ⩾400 ng/mL.9 The advent of immune checkpoint inhibitors (ICIs) has revolutionized the management of advanced HCC with the combination of atezolizumab–bevacizumab becoming the new front-line standard of care and durvalumab plus tremelimumab recently qualifying as another active front-line regimen.10 Efficacy and safety data of the treatment options proven effective for advanced HCC are summarized in Tables 1 and 2.
Table 1.
Efficacy and safety of first-line treatment options.
| SHARP4 | REFLECT6 | IMbrave15011 | HIMALAYA12 | ||
|---|---|---|---|---|---|
| STRIDE | Durvalumab | ||||
| Median OS, months | 10.7 | 13.6 | 19.2 | 16.4 | 16.6 |
| HR (95% CI) | 0.69 (0.55–0.87) | 0.92 (0.79–1.06) | 0.66 (0.52–0.85) | 0.78 (0.65–0.93) | 0.86 (0.73–1.03) |
| p Value | <0.001 | – | 0.0009 | 0.0035 | 0.0674$ |
| Median PFS, months | 5.5 | 7.4 | 6.0 | 3.8 | 3.7 |
| HR (95% CI) | 0.58 (0.45–0.74) | 0.66 (0.57–0.77) | 0.66 (0.52–0.85) | 0.90 (0.77–1.05) | 1.02 (0.88–1.19) |
| p Value | <0.001 | <0.0001 | <0.001 | – | – |
| ORR per RECIST v1.1, % | 2.0 | 24.1 | 29.8 | 20.1 | 17.0 |
| Any grade TRAEs, % | 80.0 | 94.0 | 86.0 | 75.8 | 52.1 |
| Grade ⩾3 TRAEs, % | 45.0 | 75.0 | 43.0 | 25.8 | 12.9 |
| TRAEs leading to discontinuation, % | 38.0‡ | 9.0 | 10.0§ | 8.2§ | 4.1 |
| Most frequent any grade TRAEs | Diarrhea | Hypertension | Proteinuria | Rash | Increased AST |
| Hand–foot skin reaction | Diarrhea | Hypertension | Pruritis | Rash | |
| Fatigue | Decreased appetite | Increased AST | Diarrhea | Diarrhea | |
p value of superiority to sorafenib.
Due to AEs regardless of causality.
Discontinuation of both components.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TRAEs, treatment-related adverse events; 95% CI, 95% confidence interval; –, not available.
Table 2.
Efficacy and safety of second-line treatment options.
| RESORCE7 | CELESTIAL*,8 | REACH-29 | KEYNOTE-22413 | CheckMate 040$,14 | |
|---|---|---|---|---|---|
| Median OS, months | 10.6 | 10.2 | 8.5 | 13.2 | 22.8 |
| HR (95% CI) | 0.63 (0.50–0.79) | 0.76 (0.63–0.92) | 0.71 (0.53–0.95) | – | – |
| p Value | <0.0001 | 0.005 | 0.0199 | – | – |
| Median PFS, months | 3.1 | 5.2 | 2.8 | 4.9 | – |
| HR (95% CI) | 0.44 (0.36–0.55) | 0.44 (0.36–0.52) | 0.45 (0.34–0.60) | – | – |
| p Value | <0.0001 | <0.001 | <0.0001 | – | – |
| ORR per RECIST v1.1, % | 11.0 | 4.0 | 5.0 | 18.3 | 32.0 |
| Any grade TRAEs, % | 93.0 | 99.0‡ | 46.0 | 73.1 | 94.0 |
| Grade ⩾3 TRAEs, % | 50.0 | 68.0‡ | 35.0 | 26.0 | 53.0 |
| TRAEs leading to discontinuation, % | 10.0 | 16.0‡ | 11.0 | 4.8 | 18.0 |
| Most frequent any grade TRAEs | Hand–foot skin reaction | PPE | Fatigue | Fatigue | Pruritus |
| Hypertension | Hypertension | Nausea | Increased AST | Rash | |
| Diarrhea | Increased AST | Decreased appetite | Pruritus | Diarrhea |
Second line and third-line.
4 doses nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks, then nivolumab 240 mg every 2 weeks.
Regardless of causality.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PPE, palmar-plantar erythrodysesthesia; TRAEs, treatment-related adverse events; 95% CI, 95% confidence interval; –, not available.
In this review, we will discuss treatment sequencing for advanced HCC after front-line immunotherapy, encompassing the relevant clinical and molecular markers that could help disentangle the treatment choice, and highlighting the future challenges to be addressed.
Immunotherapy in advanced HCC
Preclinical rationale
Cancer development and progression have been notoriously linked with evasion of the immune response via a plethora of mechanisms including the upregulation of immune checkpoints, namely programmed death-1 (PD-1) and its ligand (PD-L1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), that prevents immune system from overactivation.15,16 By overexpressing PD-L1, tumor cells boost the negative feedback on the immune response exerted by PD-1/PD-L1 interaction, hindering the immune response against cancer-related epitopes. The liver acts as an ‘immunological gatekeeper’ modulating the immune response against a number of antigens originating from the gut, including bacterial nucleic acid, lipopolysaccharides, and toxins.17,18 Arising within this peculiar immune-tolerant microenvironment, HCC further promotes immune escape via impaired antigen presentation, dysregulation of T-cell response, and upregulation of immunosuppressive myeloid cells. Thus, it offers a fascinating immunological background that supports the use of immunotherapy either alone or in combination with other agents to address possible mechanisms of resistance.19,20
ICI monotherapy
In the front-line setting, two PD-(L)1 inhibitors, namely durvalumab and tislelizumab have recently proven to be non-inferior compared to sorafenib in terms of OS (HR 0.86; 95.67% CI, 0.73–1.03; noninferiority margin, 1.08 for durvalumab and HR 0.85, 95.003% CI, 0.712, 1.019 for tislelizumab) in the phase III HIMALAYA trial and RATIONALE-301, respectively.12,21 However, despite showing encouraging clinical activity with an objective response rate (ORR) of 15–20% and a median OS surpassing 12 months in a pretreated population in early phase clinical trials, both nivolumab and pembrolizumab, two anti-PD-1 mAbs, failed to meet their primary endpoints in their respective global phase III trials.22–25 Of note, in the phase III KEYNOTE-394 trial enrolling pretreated Asian patients, pembrolizumab performed significantly better in all the efficacy endpoints against placebo.26 Lastly, camrelizumab, another PD-1 inhibitor, revealed encouraging antitumor activity (ORR: 14.7% with a 6-month OS of 74.4%) in pretreated Chinese patients in a single-arm phase II trial.27
Dual ICI blockade
With the aim to tackle some of the mechanisms of resistance to single-agent ICIs, a number of combinations offering a co-inhibition of PD-(L)1 and CTLA-4 were evaluated. Nivolumab plus ipilimumab is currently being tested in first-line against sorafenib or lenvatinib in the phase III CheckMate 9DW trial (NCT04039607), after proving its activity in the phase I/II CheckMate 040 trial in a post-sorafenib setting.14 Of note, in the early phase study, which tested the combination in three different dosing regimens, the arm A (4 doses nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks, then nivolumab 240 mg every 2 weeks) was associated with the highest ORR of 32%, thus receiving accelerated US Food And Drug Administration (FDA) approval.28
Furthermore, in light of the promising activity seen in the phase I/II Study 22 in sorafenib-pretreated or intolerant patients, durvalumab with or without a single priming dose of tremelimumab [also known as the STRIDE regimen (tremelimumab 300 mg one dose plus durvalumab 1500 mg every 4 weeks)] was further evaluated in the phase III HIMALAYA trial against sorafenib in the first-line setting.12,29 After a median follow-up of more than 30 months, the STRIDE regimen outperformed sorafenib in terms of median OS (HR: 0.78; 96.02% CI, 0.65–0.93; p = 0.0035), the primary endpoint of the study, showing a 36-month OS rate of 30.7% versus 20.2% and a manageable safety profile.12 As such, the combination has been recently approved by the US FDA and, therefore, will implement the treatment options available in the first-line scenario.30
ICI combinations
Since the overactivation of the VEGF pathway, a hallmark of HCC, might further enhance the immune escape, PD-1 and PD-L1 inhibitors have been also evaluated in association with anti-VEGF agents and MKIs with an antiangiogenic effect.20
After showing promising results in the phase Ib GO30140 study, atezolizumab plus bevacizumab further confirmed its activity against sorafenib in treatment-naïve patients in the subsequent phase III IMbrave150 trial, reaching a median OS of 19.2 months (HR: 0.66; 95% CI, 0.52–0.85; p = 0.0009) and a median progression-free survival (PFS) of 6.9 months (HR: 0.65; 95% CI, 0.53–0.81; descriptive p < 0.001). The combination also demonstrated an ORR of 30% and a favorable safety profile.11,31 Thus, it has become the new first-line standard of care for advanced HCC.10 Similarly, the combination of the anti-PD-1 sintilimab with a bevacizumab biosimilar (IBI305) demonstrated a significant OS and PFS benefit compared to sorafenib in a phase III trial enrolling a Chinese population.32
Regarding the ICIs plus MKIs combinations, the phase III COSMIC-312 trial testing cabozantinib–atezolizumab versus sorafenib in the front-line setting met one of its dual primary endpoints, with the experimental combination yielding a significantly longer PFS (HR: 0.63; 99% CI, 0.44–0.91; p = 0.0012). However, even though an early separation of the survival curves was seen at the interim analysis, the difference in median OS did not reach the bound for statistical significance.33 Furthermore, although the clinical activity shown in an early-phase clinical trial was encouraging, in the phase III LEAP-002 trial, the combination of pembrolizumab plus lenvatinib did not meet the primary endpoints (OS and PFS) of the study against lenvatinib.34,35 Lastly, camrelizumab combined with rivoceranib confirmed the promising efficacy demonstrated in a phase II trial (ORR of 34.3%) outperforming sorafenib in both the survival outcomes (PFS: HR: 0.52, 95% CI, 0.41–0.65; one-sided p < 0.0001; and OS: HR, 0.62, 95% CI 0.49–0.80; one-sided p < 0.0001) in the front-line setting at the planned interim analysis of an international phase III trial.36,37
The reshaped therapeutic algorithm: how to inform treatment choice and sequencing
Comparison between treatment options
Over the past 5 years, the number of active first-line systemic treatment options for patients with unresectable HCC has dramatically increased, adding considerable complexity to the therapeutic scenario. Furthermore, all the phase III randomized clinical trials testing these novel options acknowledged sorafenib as the comparator arm, leaving to indirect analyses the task of informing on the most suitable front-line treatment and its best sequential strategy.
The most updated guidelines recommend atezolizumab plus bevacizumab as the standard of care for first-line unresectable HCC,3,10,38 and a number of network meta-analyses consistently supported this recommendation by ranking atezolizumab plus bevacizumab as superior to all the other treatments evaluated in the front-line setting, including lenvatinib and nivolumab.39,40 Moreover, atezolizumab plus bevacizumab led to better survival outcomes compared to lenvatinib (HR: 0.59; 95% CI, 0.46–0.75) and sorafenib (HR: 0.58, 95% CI, 0.42–0.79), according to some matched-adjusted indirect comparisons (MAICs).41,42
The lack of direct comparison also affects the refractory setting where all the available agents were compared against placebo.17,18 Some specific features of the populations enrolled in the pivotal trials (tolerance to previous sorafenib for regorafenib, baseline AFP levels ⩾ 400 ng/mL for ramucirumab, availability also as a third-line option for cabozantinib, and suitability for immunotherapy for ipilimumab–nivolumab and pembrolizumab) could provide some guidance as to how to unravel the choice in the clinical setting. However, the largely overlapping characteristics of the patients eligible for further-line treatments make the need for a formal comparison between these treatments of utmost importance. When indirectly compared, regorafenib, cabozantinib, and ramucirumab were all established as active options for pretreated patients, with ramucirumab confirming a significant advantage only in those with elevated AFP levels.39 No survival differences between these agents were found, according to two MAICs comparing cabozantinib with regorafenib and ramucirumab, respectively.43,44 Of note, a longer median PFS was observed for cabozantinib compared with regorafenib and ramucirumab in such analyses but intrinsic differences in the trial protocols regarding the timing of the assessments could have influenced these results.43,44
Biomarkers of response
The introduction of immunotherapy in the treatment scenario represented a paradigm shift in HCC care, showing a completely new response pattern as compared to MKIs. Among responders, the treatment benefit appears durable over time, even when only the PD-1/PD-L1 pathway is targeted.13,22 On the other side, a sizeable group of patients are primary progressors, particularly when immunotherapy is not paired with antiangiogenic agents. Therefore, identifying biomarkers able to inform the best treatment approaches will be key to plan the continuum of care for such patients by a greater comprehension of the mechanisms of action and resistance to the available drugs.
The most extensively studied predictive biomarkers for immunotherapy, namely PD-L1 expression, microsatellite instability (MSI), and tumor mutational burden (TMB) are infrequently found in HCC and their correlation with tumor response remains largely unclear.45–47 For HCC patients, the PD-L1 expression was not a mandatory entry criterion to access immunotherapy nor a preplanned stratification factor. Therefore, it was not uniformly reported across clinical trials with ICIs and substantial inter-assay heterogeneity in the assessment of PD-L1 expression was observed.48 Moreover, apart from a significant association of PD-L1 combined positive score ⩾ 1 with tumor response in an exploratory analysis of the KEYNOTE-224 trial, overall similar response rates were found in both PD-L1-positive and -negative patients in trials with ICIs.13,14,31 Of note, a genomic signature of pre-existing immunity, including high expression of PD-L1 mRNA, was recently found to be significantly associated with better outcomes with a trend toward higher tumor responses as the PD-L1 cutoffs increase (⩾1, 5 or 10%) in a translational study conducted on the GO30140 and IMbrave150 data.49 Although more consistent data are being generated, the wide variability of the PD-L1 expression resulting from the retrospective nature of such analyses, the lack of standardization of the assays employed, and the different scoring systems adopted across the trials limit the usefulness of PD-L1 as a predictive biomarker in this setting and require a more rigorous systematic evaluation.48
Mismatch repair deficiency (dMMR) or MSI and TMB-high are additional, agnostic predictive biomarkers of immunotherapy benefit across solid tumors.46,47,50 In HCC, the dMMR signature has been correlated with better prognosis but the MSI-high status is overall rare (less than 3%).51 Similarly, a TMB-high is found in nearly 5% of the cases but its association with the efficacy outcomes in HCC remains poorly defined.52 Across a cohort of patients treated with atezolizumab–bevacizumab, the median TMB was around 5 mutations/Mb and tumor response was significantly associated with a TMB-high status in the phase Ib study but not in the IMbrave150 trial, according to a retrospective analysis.49 However, the relatively low prevalence of the MSI-high and TMB-high signatures in HCC limits their utility as predictors of response.46,47,53
A great effort has been made to characterize some genomic, transcriptomic, and epigenetic signatures to postulate further potential biomarkers of response to immunotherapy.52,54 High levels of immune cell infiltration, hyperexpression of PD-1 and/or PD-L1, activation of interferon-gamma (IFN-γ) signaling, and the absence of CTNNB1 mutations – a gene that encodes for β-catenin – might predict an immune-active phenotype. On the other hand, the overactivation of transforming growth factor-beta and WNT/β-catenin signaling has been linked to an immune exhausted and immune excluded subtype, respectively. Nevertheless, similar survivals were observed in patients with CTNNB1 wild-type or mutations in the IMbrave150 trial, suggesting that the addition of bevacizumab might overcome this mechanism of resistance to ICIs.49
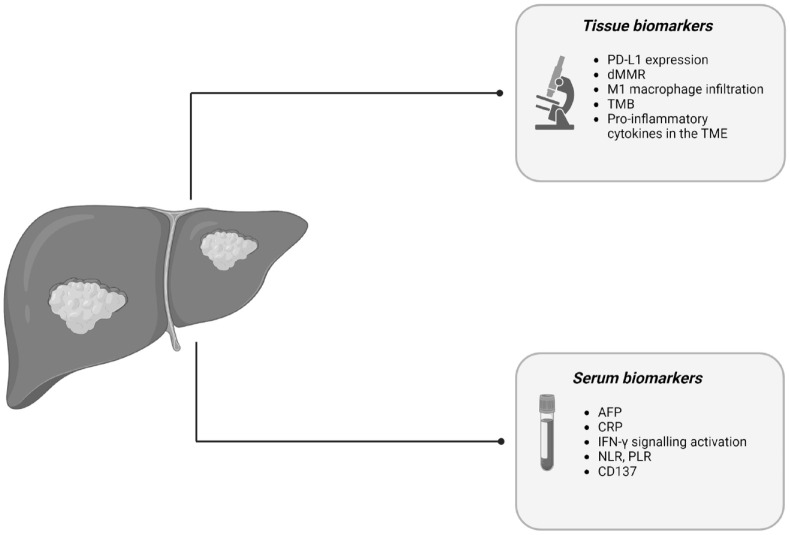
Moreover, the identification of an HCC immune gene signature failed to anticipate a clinical response to such agents.54 The understanding of the complex dynamic interactions among cancer cells, immune cells, and other immunomodulators in the tumor microenvironment (TME) will be essential to distinguish between active and exhausted or excluded immune subclasses, phenotypes that could benefit from different therapeutic approaches.54,55 Other explored correlates of response to ICIs encompass cell surface protein expression, serum and stromal biomarkers, and even certain gut microbiome richness.56 With this respect, a high serum concentration of the tumor necrosis factor family member CD137 and a high density of antitumoral M1 macrophage infiltration in the tumor stroma recently emerged as potential predictive biomarkers for the PD-1 inhibitor and anti-VEGF combination.57 Further insights might be provided by a deeper understanding of the inflammatory milieu of liver cirrhosis in which HCC typically arises. An inflammatory signature has been correlated with an immune-enriched genomic profile and response to anti-PD-1 inhibitors.52,54,58 However, the richness of pro-inflammatory cytokines in the TME fosters tumor-promoting signals and facilitates immune evasion via T-cell exhaustion and the promotion of M2-type macrophages, which ultimately is associated to a state of systemic inflammation and resistance to ICIs.59 Different immune scores tried to recapitulate this state of systemic inflammation into novel biomarkers of potential clinical utility. The CRAFITY score was developed from a combination of baseline AFP and C-reactive protein levels, with lower levels being associated with better survival and radiological responses in patients receiving anti-PD-(L)1 agents.60 Similarly, raised baseline neutrophil–lymphocyte ratio and platelet–lymphocyte ratio, reflecting a pro-inflammatory microenvironment, which promotes neoangiogenesis and metastases development, were found to retain negative prognostic significance.59,61,62
The most relevant biological determinants of response to ICIs are summarized in Figure 1.
Figure 1.
Biological determinants influencing ICIs response in advanced HCC.
Source: Created with BioRender.com.
AFP, alpha-fetoprotein; CRP, C-reactive protein; dMMR, mismatch repair-deficiency; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; IFN-γ, interferon-gamma; NLR, neutrophil–lymphocyte ratio; PD-L1, programmed death-ligand 1; PLR, platelet–lymphocyte ratio; TMB, tumor mutational burden; TME, tumor microenvironment.
Role of clinical features and drug safety profile
In the absence of validated molecular biomarkers of response, understanding the possible association of some clinical features with a potential benefit from immunotherapy is gaining certain recognition in the HCC research field. Although the treatment benefit across clinical subgroups or etiologies of the underlying liver disease was generally homogeneous across the phase III clinical trials with ICIs, some evidence suggests that the non-viral etiologies might derive less benefit from immunotherapy.63–65 According to a meta-analysis evaluating more than 1600 advanced HCC patients, treatment with PD-(L)1 inhibitors was not associated with a significant survival improvement in patients with non-viral HCC, and nonalcoholic steatohepatitis (NASH)-related HCC independently predicted unfavorable outcomes.64 Indeed, an accumulation of exhausted, dysfunctional CD8+ T cells might be responsible for this impaired immune surveillance in NASH-related HCC, according to a preclinical study.64
Furthermore, it has been suggested that immunotherapy benefit could be paired with the occurrence of immune-related adverse events (irAEs). The development of grade ⩾2 irAEs has successfully identified a subgroup of HCC patients more likely to derive better survival outcomes whilst on ICIs.66 That treatment-related adverse events (TRAEs) could predict treatment benefit is no new concept in HCC, where a wide range of side effects have been linked to MKI benefit.67–70 However, TRAEs appear of limited utility in the clinical setting as to assisting the treatment decision, not allowing an upfront selection of the optimal candidates for ICIs. Moreover, the promotion of a personalized therapeutic choice could not disregard the different safety profiles of ICIs and MKIs, and the development of severe AEs with a certain drug class should be carefully considered when the switch to a further-line option is planned.71 Increased risk of bleeding, autoimmune conditions requiring immunosuppressive treatment, and liver transplantation can limit the use of atezolizumab–bevacizumab, limiting the choice to sorafenib or lenvatinib as first-line treatments.10,72 With this respect, the future availability of antiangiogenic-free regimens, such as durvalumab with or without tremelimumab, will possibly widen the proportion of patients eligible for first-line treatment with ICIs allowing also the inclusion of those with significant cardiovascular comorbidities.12 Moreover, real-life studies could add precious information to the decision-making process, providing evidence that a certain treatment might be suitable for a broader population than that enrolled in clinical trials, as recently shown by the reassuring safety of atezolizumab–bevacizumab in Child–Pugh B patients.73
Treatment sequences: potential approaches and unsolved challenges
The unprecedented number of therapeutic choices available for advanced HCC has made remarkably more complex the selection of the most adequate sequence of treatments for each patient. The approval of a considerable number of active treatments and the availability of sequential lines of therapy led to an unprecedented survival prolongation from just over 6 months in the pre-sorafenib era to more than 2 years for patients suitable to multiple lines of therapy, and the outcomes will be probably even better for patients receiving the novel immunotherapy-based front-line options.4,74–76 Yet, after disease progression (PD) on a front-line regimen, less than 50% of HCC patients treated with ICI-based approaches receive further lines of treatment.12,24,31 Furthermore, all second- and further-line treatment options have been evaluated after sorafenib, whereas for patients who do not have contraindications, atezolizumab plus bevacizumab embodies now the front-line recommended treatment worldwide, and durvalumab with a single priming dose of tremelimumab represents a valid alternative option.3,10,38
MKIs after first-line immunotherapy-based treatments
Since no published prospective data are available so far, upon progression to ICIs, the choice of the most appropriate sequential treatment for the patients who are fit enough to receive it remains essentially empirical. In the absence of evidence-based interventions, patients’ clinical features, tolerability of the prior therapy, and labeling and regulatory approvals in each country drive the decision-making process. Enrollment within a clinical trial is warmly encouraged, where available, and several studies are currently interrogating the role of single-agent MKIs after immunotherapy, a widespread approach in this setting (e.g. NCT05134532, NCT04435977, NCT04316182). However, if a clinical study is not accessible, there exists initial reassuring evidence regarding the use of MKIs after ICIs in routine clinical practice. Both sorafenib and lenvatinib showed to be effective options after atezolizumab plus bevacizumab with a tolerability profile in keeping with the literature data, according to a real-world retrospective analysis and a simulation model built on the data from pivotal phase III trials.77,78 Interestingly, lenvatinib and sorafenib appeared as the most effective options in second line and the sequence sorafenib–cabozantinib after atezolizumab plus bevacizumab reached a median OS of 28 months in the previously mentioned simulation model.78,79 Similarly, ramucirumab, regorafenib, and cabozantinib were proven effective and safe following immunotherapy, also when received beyond the second line of therapy.80–82 Of note, ramucirumab was offered to a sorafenib-naïve population with baseline AFP levels ⩾400 ng/mL, 64% of whom received a prior line of therapy containing ICIs, whereas regorafenib and cabozantinib were given to a sorafenib-experienced population, 9% and 10% of whom, respectively, received prior immunotherapy. Therefore, either shifting forward sorafenib or lenvatinib, originally approved in the first-line setting, or adopting regorafenib, cabozantinib, or ramucirumab, approved as further-line options after sorafenib, are acceptable strategies recognized by most of the international guidelines.3,10,38,83
Immunotherapy beyond radiological progression
Maintaining treatments beyond progression in case of sustained clinical benefit might be a reasonable strategy with ICIs, a class of agents recognized for inducing unique patterns and timing of response. Delayed response and pseudoprogression are renowned phenomena in immune-oncology, although rather infrequent in the HCC field.84–86 Indeed, around 30–60% of HCC patients show primary resistance to ICIs within clinical trials.12,14,24,25,29 However, even in patients displaying PD as best response, ICIs could lead to unconventional benefit. In the IMbrave150 trial, more than half of the patients continued to receive atezolizumab plus/minus bevacizumab beyond PD with reported sustained benefit and prolonged survival.11 A similar trend was observed in the HIMALAYA trial, where slightly less than half of the patients on an ICI-containing arm continued to receive treatment beyond PD, and 7.6% of the patients in the STRIDE arm were rechallenged with a second priming dose of tremelimumab at PD.12 Disentangling in a timely fashion the subgroup of patients among progressors that could still benefit from treatment will be essential, and the pattern of progression might appear as an informative parameter in doing so. With this respect, nivolumab yielded durable survival benefit reaching a median OS of 18.8 months in patients with PD of target lesions followed by a formal response, PD with new lesions followed by ⩾10% decrease in target lesions, or PD of target lesions or new lesions followed by stabilization of the disease burden, compared with 8.4 months in the remaining progressors, according to a post-hoc analysis of the CheckMate 040 study.87 Thus, the evidence of radiological PD should not always claim treatment failure and prompt to switch to a subsequent line of therapy. Although continuing treatment beyond progression is no new concept, the decision is usually made per investigator’s choice in clinical trials in the absence of well-established biomarkers of response and elucidated mechanisms of primary and acquired resistance to ICIs. Yet, while promising also in a real-world scenario, this approach seems much less adopted for unselected patients in a less rigorous setting like that of clinical practice.88
Immunotherapy rechallenge
Despite maintaining ICIs beyond progression might be considered in specific circumstances, immunotherapy discontinuation is usually advised upon tumor progression and/or after the occurrence of severe AEs in clinical trials, and limited evidence exists regarding the clinical outcomes at treatment resumption.89 So far, only very few HCC patients (<2%) receiving ICIs within phase III clinical trials have been switched to another immune-oncology agent after PD and the effects of rechallenge with immunotherapy are largely unknown.11,12,24 In the real-world setting, some evidence suggests that rechallenge with ICIs is associated with similar rates of radiological responses and irAEs compared to the first ICI-containing regimen.90,91 Indeed, ORR ranged from 16% to 26% with responses seen also in the subgroup of primary progressors and grade 3–4 irAEs ranged from 8% to 17%, according to two retrospective studies. Of note, a new line of treatment with ICIs was offered in up to 6% of patients and tumor responses were observed regardless of the regimen type (monotherapy or combinations).90
Reoccurrence of severe irAEs after ICI rechallenge, however, remains to be elucidated for HCC patients. Across other tumor types, mostly melanoma and non-small-cell lung cancer, clinically significant irAEs were reported in 18–44% of patients (both new and recurrent AEs) with immunotherapy retreatment, and higher irAE rates happened with anti-CTLA-4 agents alone or in combinations.89,92,93 In the HCC setting, no toxicity recurrence was observed across the patients retreated with nivolumab with or without ipilimumab – temporarily withheld due to irAEs – in the CheckMate 040 study.14 Furthermore, only 5% of patients rechallenged in a real-world scenario developed again grade 3–4 irAEs, although the small sample size of the study limits the conclusions that can be drawn.90 Although worth of further investigations, this strategy will serve a limited number of patients as treatment discontinuation due to irAEs did not exceed 15% for ICI combinations and 5% for ICI monotherapy in phase III trials in the front-line setting.11,12,24,33 A more relevant issue might be posed by the subgroup of patients experiencing severe toxicity on combinations of ICIs with either anti-VEGF mAb or MKIs. Despite the treatment discontinuation rate is not much different from those receiving ICIs alone, grade 3 or higher AEs have been reported in 43–81% of patients receiving combinations with antiangiogenics, with most of the AEs falling within the spectrum of the expected side effects of the antiangiogenic compound.11,33,35,37 Therefore, the choice of MKIs as a suitable further line of therapy – one of the most widespread approaches – would require a cautious evaluation in this setting.
For the vast majority of patients discontinuing ICIs for PD, either shifting to a different drug class with or without maintaining ICI or, in the frame of early phase clinical trials, exploring other treatment avenues with new immunotherapeutic agents (e.g. agonist immunostimulatory mAbs, bispecific antibodies, engineered cytokines, antibody–drug conjugates, adoptive T-cell therapy, and neoantigen vaccination) might represent more successful sequential strategies.
Changing companion in immunotherapy-based combinations
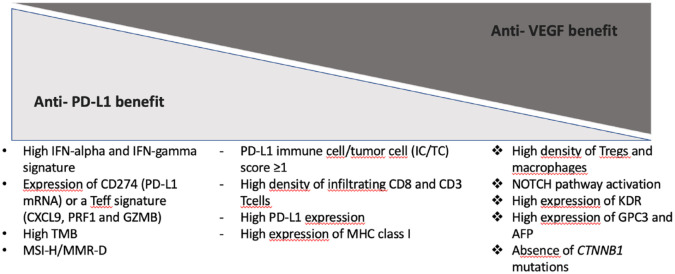
Another sequential approach considers the replacement of the anti-VEGF agent with an MKI with the hope that their broader mechanism of action could tackle some of the resistance pathways emerging during the treatment with atezolizumab plus bevacizumab. A number of clinical trials are questioning the added value of maintaining the ICI compound after PD when its anti-VEGF companion is substituted with an MKI (e.g. NCT04770896, NCT05168163). Further endorsing the relevant preclinical rationale that supported the development of combinations with ICIs and antiangiogenics, recent translational evidence shed light on some biological mechanisms driving the augmented antitumor activity seen co-targeting PD-(L)1 and the VEGF pathway in the clinical setting.49 Indeed, PD rates were much lower with atezolizumab–bevacizumab (19%) and cabozantinib–atezolizumab (14%) as compared to nivolumab (37%) or durvalumab with (40%) or without tremelimumab (45%).11,12,33 However, it remains to be elucidated whether or not the use of ICI-MKI combinations would restore sensitivity to immunotherapy even in the refractory setting by reigniting an immunosuppressive microenvironment, particularly for patients not previously exposed to antiangiogenics in the front-line setting. Furthermore, pairing ICIs with MKIs would take advantage of a wider inhibition of several tyrosine kinase-mediated pathways – dysregulated in HCC development and progression – than targeting angiogenesis alone, hopefully reestablishing ICI susceptibility also in patients previously exposed to anti-VEGF agents. Moreover, these ongoing studies will also provide further hints on the optimal MKI to be used in the sequential strategy. A summary of the ongoing clinical trials for each proposed sequential approach is provided in Table 3 while the most relevant biological predictors of benefit from ICIs alone and combined with antiangiogenics are summarized in Figure 2.
Table 3.
Sequential strategies after immunotherapy explored in clinical trials.
| Sequential strategy | Trial name/number | Phase/design | Regimens | Primary endpoint |
|---|---|---|---|---|
| Switching to MKIs | REGONEXT (NCT05134532) | Phase II – single arm, open label | Regorafenib after atezolizumab–bevacizumab | PFS |
| Immunocabo (NCT04435977) | Single arm, open label | Cabozantinib after ICIs | PFS | |
| ACTION (NCT04316182) | Phase II – single arm, open label | Cabozantinib in sorafenib intolerant or after non-sorafenib-based first-line treatment | TTP | |
| Switching to MKIs maintaining ICIs | IMbrave251 (NCT04770896) | Phase III – randomized, open label | Atezolizumab plus lenvatinib or sorafenib versus lenvatinib or sorafenib alone | OS |
| NCT05168163 | Phase II – randomized, open label | Atezolizumab plus MKIs (cabozantinib or lenvatinib) versus MKIs alone after ICIs | PFS and OS | |
| NCT05101629 | Phase II – single arm, open label | Pembrolizumab plus lenvatinib after ICIs | ORR | |
| NCT04696055 | Phase II – non-randomized, open label | Pembrolizumab plus regorafenib following anti-PD(L)1 therapy | ORR | |
| GOING (NCT04170556) | Phase I/II – single arm, open label | Regorafenib followed by nivolumab from week 8 after progression on sorafenib (cohort A) or atezolizumab–bevacizumab (cohort B) | Safety | |
| ICIs beyond progression* | IMbrave150 (NCT03434379) | Phase III – randomized (2:1), open label | Atezolizumab plus bevacizumab versus sorafenib in treatment-naïve HCC patients | PFS and OS |
| HIMALAYA (NCT03298451) | Phase III – randomized (1:1:1), open label | Durvalumab plus or minus tremelimumab versus sorafenib in treatment-naïve HCC patients | OS | |
| CheckMate 459 (NCT02576509) | Phase III – randomized (1:1), open label | Nivolumab versus sorafenib in treatment-naïve HCC patients | OS | |
| Novel immunotherapies | NCT04374877 | Phase I – open label | SRF388, a IgG1 antibody against IL-27, alone or in combination with pembrolizumab in advanced solid tumors including HCC | Safety and ORR |
| NCT05003895 | Phase I – single arm, open label | GPC3 targeted CAR-T Cell therapy in advanced GPC3+ HCC | Safety and feasibility | |
| NCT05070156 | Early phase I – single arm, open label | B010-A Injection, a neoantigen vaccine, in advanced GPC3+ HCC | Safety | |
| NCT05293496 | Phase I/Ib – open-label | MGC018, an ADC against B7-H3$, in combination with MGD019, a bispecific DART molecule that binds PD-1 and CTLA-4, in advanced solid tumors including HCC | Safety |
Phase III trials allowing treatment with ICIs beyond progression in the front-line setting.
B7-H3 is an additional immune checkpoint potentially involved in resistance to anti-PD-1/PD-L1 blockade.
ADC, antibody–drug conjugate; B7-H3, B7 Homolog 3; CTLA-4, Cytotoxic T lymphocyte-associated antigen-4; DART, dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors; GPC3, glypican-3; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; IgG1, immunoglobulin G1, IL-27, interleukin-27; MKIs, multikinase inhibitors; ORR, objective response rate; OS, overall survival; PD(L)-1, programmed death (ligand)-1; PFS, progression-free survival; TTP, time to progression.
Figure 2.
Biological predictors of benefit from ICIs alone and combined with antiangiogenics.
AFP, alpha-fetoprotein; ICIs, immune checkpoint inhibitors; IFN-alpha, interferon alpha; IFN-gamma, interferon-gamma; MHC, major histocompatibility complex; MMR-D, mismatch repair-deficiency; mRNA, messenger ribonucleic acid; MSI-H, microsatellite instability-high; PD-L1, programmed death-ligand 1; TMB, tumor mutational burden; Tregs, regulatory T cells; VEGF, vascular endothelial growth factor.
Conclusions and future directions
After more than a decade of sorafenib monopoly, the therapeutic scenario for advanced HCC has grown at a sustained pace, leading to the approval of novel therapies and to an unprecedented survival prolongation in this setting. However, the advancement in terms of drug development has not been followed by a systematic understanding of how to redesign the therapeutic landscape. In the absence of head-to-head comparisons between these regimens nor validated predictive biomarkers other than elevated baseline AFP levels for ramucirumab, considerable uncertainty remains as to the most appropriate treatment choice.9,94 Thus, nowadays, treatment selection and sequencing are still predominantly guided by a comprehensive assessment of patient and treatment characteristics altogether with regulatory approvals.
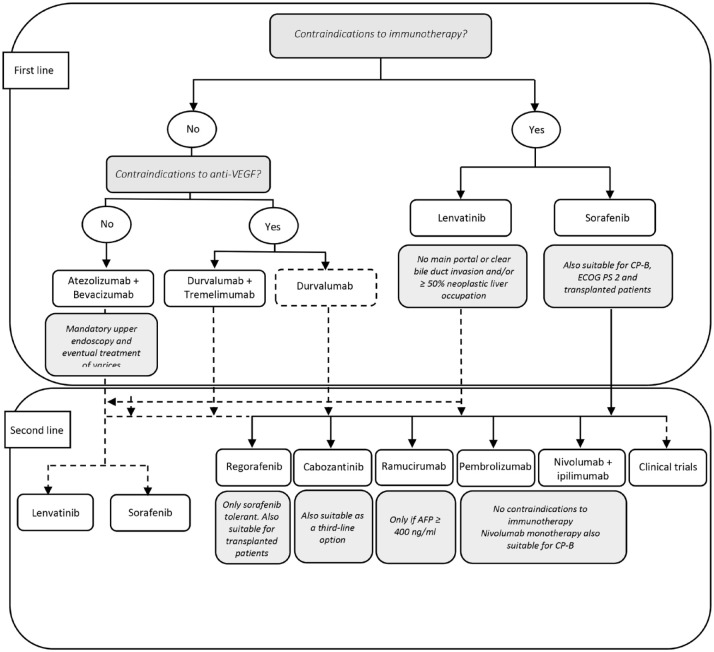
The entry of ICI-based combinations into the front-line armamentarium has imposed a thorough reflection on how to unravel the increased number of potential treatment sequences (Figure 3). Indeed, informed strategies for patients that have not been exposed to prior sorafenib remain to be elucidated. When offered after immunotherapy, preliminary data suggest that MKIs retain a drug profile in keeping with the literature data.77,78 On the other side, when retreatment with ICIs is considered, balancing the potential benefits derived from the restored treatment efficacy with the concerns of irAE reoccurrence is crucial. Therefore, enrollment within a clinical trial or treatment in an experienced clinical setting should be endorsed, lacking rigorous evidence that supports this choice.
Figure 3.
Current treatment algorithm and potential alternative sequences.
Furthermore, the criteria themselves guiding the shift to a further line of therapy are being revisited in certain circumstances. As for other cancer types, initial evidence suggests that maintaining treatment with ICIs beyond radiological disease progression could be an option in carefully selected patients as the survival benefit might not be always accurately recapitulated by the radiological metrics in immune-oncology.84–86 Nevertheless, this approach needs further validation before a wider use in clinical practice could be advised as not switching to a further line of treatment in a timely manner might affect patient eligibility for further active treatments due to the concomitant potential deterioration of liver function. Hopefully, the ongoing clinical trials testing MKIs – as single agents or in combinations with ICIs – and novel immunotherapeutic strategies will generate data on the most effective sequential treatments after tumor progression on ICIs and provide some hints on how to optimize patient selection.
Despite the identification of different molecular subclasses of HCC has thrown light on some genetic, epigenetic, and immunological features that could act as potential predictors of response, precision medicine in HCC has not been a particularly successful path to follow so far, leaving behind the set-up of biomaker-enriched clinical trials to favor all-comers study designs.95–97 Inappropriate target, patient, and treatment selection, intra and inter-tumor heterogeneity might have contributed to the failure of such tailored approaches. Furthermore, the limited number of correlative studies due to the paucity of available tumor samples clearly represented a major barrier in the understanding of the leading mechanisms of treatment response or resistance in this setting. In fact, particularly in the advanced stage where a radiological diagnosis does not necessarily require further histological confirmation in cirrhotic patients, the reduced number of tumor biopsies has hampered the discovery and validation of predictive biomarkers of clinical utility that could inform treatment choice at each time point.54,98 With this respect, the studies evaluating neoadjuvant and perioperative approaches with ICIs will represent a privileged field for biomarkers discovery and validation.99–101 Indeed, the availability of longitudinal, prospectively collected tissue samples will likely enable a comprehensive evaluation of the correlates of response to such treatments at a biological level that could possibly be informative also for the advanced stage.
While tumor tissue remains the optimal source for identifying tumor-specific biomarkers, liquid biopsy is emerging as an attractive, non-invasive procedure that could overcome the matter of reduced tissue availability, capture tumor heterogeneity, and offer longitudinal treatment monitoring without the need for repeated tumor sampling.102 Compared to other malignancies, still fewer data are available for HCC, but cell-free DNA, cell-free RNA, extracellular vesicles, and circulating tumor cells all appear promising candidates for identifying a biomarker of interest and tracking on-treatment tumor changes.103–106 Prospective longitudinal studies have identified some predictive mutational signatures associated with responses to MKIs using circulating tumor DNA. However, the detection of activating mutations in WNT/β-catenin pathway as a feature associated with resistance to ICIs – as shown in previous studies on tissue samples – has not been confirmed with liquid biopsy so far.107
Moving forward, multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics offer integrative insights into the molecular mechanisms of HCC development and progression, possibly suggesting reliable tumor biomarkers that could inform treatment selection and sequencing in clinical practice.108 Even a certain gut microbiome richness is emerging as an intriguing predictor of response or resistance to ICIs and there is increasing interest in evaluating the modulation of the microbiome as a mechanism to regulate the immune composition of the TME.109 Different plasma proteins and miRNAs with a role in HCC development, tyrosine kinase receptor signaling, and tumor angiogenesis were found to retain prognostic and sometimes predictive significance in retrospective analyses of pivotal trials with MKIs, despite being of limited clinical value in the therapeutic decision-making, lacking of further thorough validation.110–115 For patients with HCC receiving immunotherapy, PD-L1 expression and TMB levels correlated poorly with clinical outcomes.45–47 In its place, a genomic signature of pre-existing immunity, including high expression of PD-L1 mRNA, enrichment of inflammation response pathways, and high density of CD8+ T cells in the TME successfully predicted response to atezolizumab plus bevacizumab in a recent study, pointing out that a comprehensive assessment of the dynamic interactions between tumor cells and the TME can more accurately capture susceptibility to immunotherapy.49 Interestingly, the addition of bevacizumab to atezolizumab was found to enhance the antitumor activity of the anti-PD-L1 agent by targeting VEGF-mediated angiogenesis, regulatory T cells proliferation, and myeloid cell inflammation, all features that promote resistance to ICIs. Therefore, further validation of these translational findings and their integration with the upcoming evidence from the ongoing studies and the relevant clinical data for each subject will hopefully help determine enriched subsets of patients that could benefit from a specific treatment at each stage with the least toxicity, ultimately guiding the decision-making in routine practice.
Acknowledgments
D.J. Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre.
Footnotes
ORCID iDs: Antonella Cammarota  https://orcid.org/0000-0001-9967-4694
https://orcid.org/0000-0001-9967-4694
Lorenza Rimassa  https://orcid.org/0000-0001-9957-3615
https://orcid.org/0000-0001-9957-3615
Contributor Information
Antonella Cammarota, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (Milan), Italy; Drug Development Unit, Sarah Cannon Research Institute UK, London, UK.
Valentina Zanuso, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (Milan), Italy; Medical Oncology and Hematology Unit, IRCCS Humanitas Research Hospital, Rozzano (Milan), Italy.
Giulia Francesca Manfredi, Division of Internal Medicine, Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy.
Ravindhi Murphy, Department of Surgery & Cancer, Imperial College London, London, UK.
David James Pinato, Department of Surgery & Cancer, Imperial College London, London, UK; Division of Oncology, Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy.
Lorenza Rimassa, Department of Biomedical Sciences, Humanitas University, Via Rita Levi Montalcini 4, 20072 Pieve Emanuele (Milan), Italy; Medical Oncology and Hematology Unit, IRCCS Humanitas Research Hospital, 20089 Rozzano (Milan), Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Antonella Cammarota: Conceptualization; Data curation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Valentina Zanuso: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Giulia Francesca Manfredi: Data curation; Methodology; Writing – original draft.
Ravindhi Murphy: Data curation; Methodology; Writing – original draft.
David James Pinato: Conceptualization; Data curation; Methodology; Supervision; Writing – review & editing.
Lorenza Rimassa: Conceptualization; Data curation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
DJ Pinato has received lecture fees from ViiV Healthcare and Bayer HealthCare; payments or honoraria from BMS, Roche, Eisai, and the Falk Foundation; travel expenses from BMS and Bayer HealthCare; consulting fees from Mina Therapeutics, Eisai, Roche, AstraZeneca, Da Volterra, and BMS; and research funding (to his institution) from MSD and BMS. L Rimassa has received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi, Servier; travel expenses from AstraZeneca; and research funding (to her institution) from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. The other authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Availability of data and materials: The data used in writing this review are reported in the references.
References
- 1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis, prediction and treatment recommendation: the 2022 update. J Hepatol 2022; 76: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 5. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 8. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 282–296. [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network. Hepatobiliary cancers. Version 1, https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (2022, accessed 1 July 2022).
- 11. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022; 76: 862–873. [DOI] [PubMed] [Google Scholar]
- 12. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 2022; 1: EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 13. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 14. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 2020; 6: e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 2020; 17: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- 18. Mima K, Nakagawa S, Sawayama H, et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett 2017; 402: 9–15. [DOI] [PubMed] [Google Scholar]
- 19. Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: current and future. World J Gastroenterol 2019; 25: 2977–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: looking outside the box. J Hepatol 2020; 72: 342–352. [DOI] [PubMed] [Google Scholar]
- 21. Qin S, Kudo M, Meyer T, et al. Final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022; 33: S808–S869. [DOI] [PubMed] [Google Scholar]
- 22. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laethem JLV, Borbath I, Karwal L, et al. Pembrolizumab (pembro) monotherapy for previously untreated advanced hepatocellular carcinoma (HCC): phase II KEYNOTE-224 study. J Clin Oncol 2021; 39: 297–297. [Google Scholar]
- 24. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022; 23: 77–90. [DOI] [PubMed] [Google Scholar]
- 25. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 26. Qin S, Chen Z, Fang W, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol 2022; 40: 383–383. [Google Scholar]
- 27. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020; 21: 571–580. [DOI] [PubMed] [Google Scholar]
- 28. FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma, bit.ly/3z47yZc (accessed 1 July 2022). [Google Scholar]
- 29. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol 2021; 39: 2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. FDA approves tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma, rb.gy/pl2hji (accessed 25 October 2022). [Google Scholar]
- 31. Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 2020; 21: 808–820. [DOI] [PubMed] [Google Scholar]
- 32. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021; 22: 977–990. [DOI] [PubMed] [Google Scholar]
- 33. Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022; 23: 995–1008. [DOI] [PubMed] [Google Scholar]
- 34. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020; 38: 2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finn RS, Kudo M, Merle P, et al. Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022; 33: S808–S869. [Google Scholar]
- 36. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res 2021; 27: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 37. Qin S, Chan LS, Gu S, et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol 2022; 33: S808–S869. [Google Scholar]
- 38. Vogel A, Martinelli E. and ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol 2021; 32: 801–805. [DOI] [PubMed] [Google Scholar]
- 39. Sonbol MB, Riaz IB, Naqvi SAA, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol 2020; 6: e204930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vogel A, Rimassa L, Sun HC, et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver Cancer 2021; 10: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Casadei-Gardini A, Tada T, Shimose S, et al. Is atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma superior even to lenvatinib? a matching-adjusted indirect comparison. Target Oncol 2021; 16: 249–254. [DOI] [PubMed] [Google Scholar]
- 42. Jiang Y, Cai D, Shi S. Indirect comparisons via sorafenib for the comparative effectiveness of two PD-1/PD-L1 inhibitors to treat advanced hepatocellular carcinoma patients without prior systemic therapies. Clin Epidemiol 2022; 14: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelley RK, Mollon P, Blanc JF, et al. Comparative efficacy of cabozantinib and regorafenib for advanced hepatocellular carcinoma. Adv Ther 2020; 37: 2678–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trojan J, Mollon P, Daniele B, et al. Comparative efficacy of cabozantinib and ramucirumab after sorafenib for patients with hepatocellular carcinoma and alpha-fetoprotein ⩾ 400 ng/mL: a matching-adjusted indirect comparison. Adv Ther 2021; 38: 2472–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 2021; 18: 345–362. [DOI] [PubMed] [Google Scholar]
- 46. Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020; 55: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goumard C, Desbois-Mouthon C, Wendum D, et al. Low levels of microsatellite instability at simple repeated sequences commonly occur in human hepatocellular carcinoma. Cancer Genomics Proteomics 2017; 14: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pinato DJ, Mauri FA, Spina P, et al. Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the Blueprint-HCC study. Br J Cancer 2019; 120: 1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu AX, Abbas AR, de Galarreta MR, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med 2022; 28: 1599–1611. [DOI] [PubMed] [Google Scholar]
- 50. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020; 21: 1353–1365. [DOI] [PubMed] [Google Scholar]
- 51. Zhao P, Li L, Jiang X, et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ang C, Klempner SJ, Ali SM, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019; 10: 4018–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wong CN, Fessas P, Dominy K, et al. Qualification of tumour mutational burden by targeted next-generation sequencing as a biomarker in hepatocellular carcinoma. Liver Int 2021; 41: 192–203. [DOI] [PubMed] [Google Scholar]
- 54. Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018; 15: 599–616. [DOI] [PubMed] [Google Scholar]
- 55. Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 2017; 153: 812–826. [DOI] [PubMed] [Google Scholar]
- 56. Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 2019; 7: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang W, Gong C, Peng X, et al. Serum concentration of CD137 and tumor infiltration by M1 macrophages predict the response to sintilimab plus bevacizumab biosimilar in advanced hepatocellular carcinoma patients. Clin Cancer Res 2022; 28: 3499–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol 2020; 73: 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Muhammed A, D’Alessio A, Enica A, et al. Predictive biomarkers of response to immune checkpoint inhibitors in hepatocellular carcinoma. Expert Rev Mol Diagn 2022; 22: 253–264. [DOI] [PubMed] [Google Scholar]
- 60. Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol 2022; 76: 353–363. [DOI] [PubMed] [Google Scholar]
- 61. Muhammed A, Fulgenzi CAM, Dharmapuri S, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel) 2021; 14: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dharmapuri S, Özbek U, Lin JY, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med 2020; 9: 4962–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haber PK, Puigvehí M, Castet F, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology 2021; 161: 879–898. [DOI] [PubMed] [Google Scholar]
- 64. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021; 592: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rimini M, Rimassa L, Ueshima K, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open 2022; 7: 100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pinato DJ, Marron TU, Mishra-Kalyani PS, et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer 2021; 157: 140–152. [DOI] [PubMed] [Google Scholar]
- 67. Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol 2014; 61: 318–324. [DOI] [PubMed] [Google Scholar]
- 68. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol 2018; 36: 412. [Google Scholar]
- 69. Sung MW, Finn RS, Qin S, et al. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT). J Clin Oncol 2019; 37: 317. [Google Scholar]
- 70. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Association of adverse events (AEs) with efficacy outcomes for cabozantinib (C) in patients (pts) with advanced hepatocellular carcinoma (aHCC) in the phase III CELESTIAL trial. J Clin Oncol 2019; 37: 4088. [Google Scholar]
- 71. Sangro B, Chan SL, Meyer T, et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol 2020; 72: 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rimassa L, Personeni N, Czauderna C, et al. Systemic treatment of HCC in special populations. J Hepatol 2021; 74: 931–943. [DOI] [PubMed] [Google Scholar]
- 73. D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology 2022; 76: 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alsina A, Kudo M, Vogel A, et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: a post hoc responder analysis from the phase 3 REFLECT study in unresectable hepatocellular carcinoma. Liver Cancer 2020; 9: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol 2018; 69: 353–358. [DOI] [PubMed] [Google Scholar]
- 76. Kelley RK, Ryoo BY, Merle P, et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020; 5: e000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoo C, Kim JH, Ryu MH, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer 2021; 10: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cabibbo G, Reig M, Celsa C, et al. First-line immune checkpoint inhibitor-based sequential therapies for advanced hepatocellular carcinoma: rationale for future trials. Liver Cancer 2021; 11: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Celsa C, Cabibbo G, Battaglia S, et al. Efficacy and safety of atezolizumab plus bevacizumab-based sequential treatment for unresectable hepatocellular carcinoma: a simulation model. In: EASL international liver congress 2022, London, 22–26 June 2022. Poster THU-599. [Google Scholar]
- 80. Finn RS, Yau T, Hsu CH, et al. Ramucirumab for patients with advanced hepatocellular carcinoma and elevated alpha fetoprotein following non-sorafenib systemic therapy: an expansion cohort of REACH-2. Oncologist 2022; 27: e938–e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Finn RS, Kudo M, Klümpen HJ, et al. Regorafenib in patients with unresectable hepatocellular carcinoma (uHCC) in routine clinical practice: exploratory analysis of overall survival (OS) in the prospective, observational REFINE study. J Clin Oncol 2022; 40: 433.34882501 [Google Scholar]
- 82. Abou-Alfa G, Cheng A-L, Saletan S, et al. Clinical activity of cabozantinib in patients with advanced hepatocellular carcinoma previously treated with anti-VEGF and immuno-oncology therapy: subgroup analysis from the phase 3 CELESTIAL trial. In: EASL Liver Cancer Summit 2020, Prague, 6-8 February 2020. Poster Blast PB02-04. [Google Scholar]
- 83. Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol 2021; 75: 960–974. [DOI] [PubMed] [Google Scholar]
- 84. Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015; 33: 3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol 2018; 58: 125–135. [DOI] [PubMed] [Google Scholar]
- 86. Manitz J, D’Angelo SP, Apolo AB, et al. Comparison of tumor assessments using RECIST 1.1 and irRECIST, and association with overall survival. J ImmunoTher Cancer 2022; 10: e003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. El-Khoueiry AB, Melero I, Yau TC, et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): subanalyses of CheckMate-040. J Clin Oncol 2018; 36: 475. [Google Scholar]
- 88. Talbot T, D’Alessio A, Pinter M, et al. Progression patterns and therapeutic sequencing following immune checkpoint inhibition for HCC: an observational study. In: EASL Liver Cancer Summit 2022, Virtual Conference, 3–4 February 2022. Poster PO–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao Q, Zhang J, Xu L, et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol 2021; 12: 730320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scheiner B, Roessler D, Phen S, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep. Epub ahead of print October 2022. DOI: 10.1016/j.jhepr.2022.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wong JSL, Kwok GGW, Tang V, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer 2021; 9: e001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 2019; 5: 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 2020; 6: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fulgenzi CAM, D’Alessio A, Ogunbiyi O, et al. Novel immunotherapy combinations in clinical trials for hepatocellular carcinoma: will they shape the future treatment landscape? Expert Opin Investig Drugs 2022; 31: 681–691. [DOI] [PubMed] [Google Scholar]
- 95. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018; 19: 682–693. [DOI] [PubMed] [Google Scholar]
- 96. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013; 31: 3517–3524. [DOI] [PubMed] [Google Scholar]
- 97. Zhu AX, Rosmorduc O, Evans TRJ, et al. Search: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015; 33: 559–566. [DOI] [PubMed] [Google Scholar]
- 98. Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 2020; 72: 215–229. [DOI] [PubMed] [Google Scholar]
- 99. Marron TU, Fiel MI, Hamon P, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022; 7: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kaseb AO, Hasanov E, Cao HST, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022; 7: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. D’Alessio A, Pai M, Spalding D, et al. PRIME-HCC: phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. In: EASL international liver congress 2022, London, 22–26 June 2022. Oral presentation-OS158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discov 2021; 11: 858–873. [DOI] [PubMed] [Google Scholar]
- 103. Labgaa I, Villacorta-Martin C, D’Avola D, et al. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene 2018; 37: 3740–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pelizzaro F, Cardin R, Penzo B, et al. Liquid biopsy in hepatocellular carcinoma: where are we now? Cancers (Basel) 2021; 13: 2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Moldogazieva NT, Zavadskiy SP, Terentiev AA. Genomic landscape of liquid biopsy for hepatocellular carcinoma personalized medicine. Cancer Genomics Proteomics 2021; 18: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology 2021; 160: 2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. von Felden J, Craig AJ, Garcia-Lezana T, et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene 2021; 40: 140–151. [DOI] [PubMed] [Google Scholar]
- 108. Chen F, Wang J, Wu Y, et al. Potential biomarkers for liver cancer diagnosis based on multi-omics strategy. Front Oncol 2022; 12: 822449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schneider KM, Mohs A, Gui W, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun 2022; 13: 3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Teufel M, Seidel H, Köchert K, et al. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology 2019; 156: 1731–1741. [DOI] [PubMed] [Google Scholar]
- 111. Llovet JM, Peña CEA, Lathia CD, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012; 18: 2290–2300. [DOI] [PubMed] [Google Scholar]
- 112. Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of sorafenib efficacy in advanced hepatocellular carcinoma: are we getting there? World J Gastroenterol 2015; 21: 10336–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rimassa L, Abbadessa G, Personeni N, et al. Tumor and circulating biomarkers in patients with second-line hepatocellular carcinoma from the randomized phase II study with tivantinib. Oncotarget 2016; 7: 72622–72633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rimassa L, Kelley RK, Meyer T, et al. Outcomes based on plasma biomarkers for the phase 3 CELESTIAL trial of cabozantinib versus placebo in advanced hepatocellular carcinoma. Liver Cancer 2022; 11: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Finn RS, Kudo M, Cheng AL, et al. Pharmacodynamic biomarkers predictive of survival benefit with lenvatinib in unresectable hepatocellular carcinoma: from the phase III REFLECT study. Clin Cancer Res 2021; 27: 4848–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]