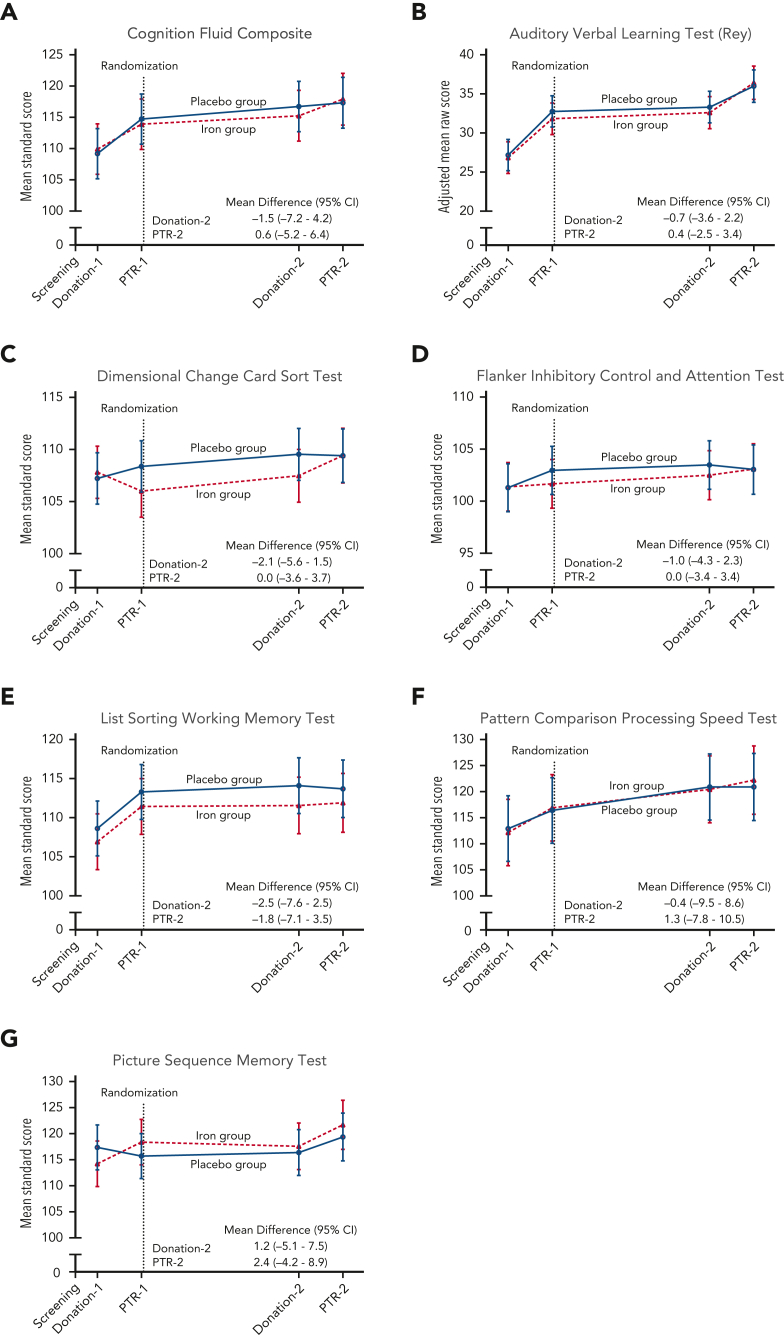
Figure 4.
Cognitive performance measurements during the trial. The data points represent the estimated means based on a mixed-model repeated-measures analysis after adjustment for the baseline value. The vertical bars denote 95% CIs. The dependent variable was the cognitive test score at each predetermined time point. Fixed effects included the interaction between treatment and time. Time was treated as a categorical variable. The subject was included in the model as a random effect. A first-order autoregressive covariance matrix was used to model the within-patient variance-covariance errors. The primary outcome was the (A) Cognition Fluid Composite Score. Prespecified secondary outcomes were the (B) raw score on the Auditory Verbal Learning Test (Rey), (C) Dimensional Change Card Sort Test, (D) Flanker Inhibitory Control and Attention Test, (E) List Sorting Working Memory Test, (F) Pattern Comparison Processing Speed Test, and (G) Picture Sequence Memory Test. Standard scores have a mean of 100 and SD of 15, with higher scores representing better cognitive performance. PTR, posttransfusion recovery.