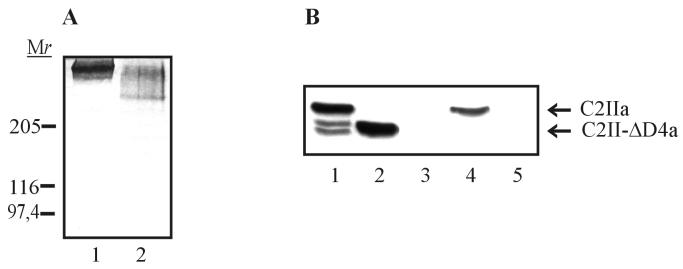
FIG. 5.
Characterization of C2II-ΔD4. C2II-ΔD4 was expressed as a GST fusion protein in E. coli and cleaved with thrombin from glutathione-Sepharose beads. (A) C2II-ΔD4 and C2II were activated with trypsin and subjected to SDS–3 to 12.5% PAGE without prior heating and stained with Coomassie blue. Lane 1, C2IIa oligomer; lane 2, C2II-ΔD4a oligomer. Relative molecular weights (in thousands) are noted. (B) Binding of truncated C2IIa to NIH 3T3 cells. Two hundred nanograms of C2IIa and 200 ng of C2II-ΔD4a were separately added to prechilled NIH 3T3 cells in 1 ml of ice-cold HBSS. Cells were incubated for 1.5 h on ice, washed five times with ice-cold PBS, and lysed in 25 mM HEPES–2 mM MgCl2. Proteins were subjected to SDS–12.5% PAGE and analyzed by Western blotting with anti-C2II182–196 antiserum. Lane 1, C2IIa; lane 2, C2II-ΔD4a; lane 3, control cells; lane 4, cells incubated with C2IIa; lane 5, cells incubated with C2II-ΔD4a.